Translate this page into:
Transthyretin Amyloid Cardiomyopathy in a Young Female
Sama Rajashekhar, DM Department of Cardiology, Nizam's Institute of Medical Sciences Hyderabad, Telangana 500082 India Samarajashekharreddy121@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Discussant: Dr. Sama Rajashekhar
Presenter: An 18 years old female patient came with history of difficulty in breathing on exertion, initially New York Heart Association (NYHA) Functional Classification (FC) II for 6 months, progressed to NYHA FC III over the next 3 months. There was history of paroxysmal nocturnal dyspnea (PND) attacks and pedal edema for the last 2 months. Patient was taken to outside hospital where she was managed conservatively and symptoms improved. On presentation to our hospital she had shortness of breath (SOB) NYHA FC II.
She did not complain of chest pain, palpitations, abdominal distention, facial puffiness, reduced urine output, fever, generalized weakness, cough with or without expectoration, loss or gain in weight, skin rash, pain or swelling of joints, generalized anasarca, pain abdomen, constipation, diarrhea, melena, burning micturition, tingling numbness of limbs, and bleeding manifestations.
There were no similar complaints in the past. She takes both vegetarian and nonvegetarian diet. No history of consumption of alcohol or tobacco in any form. Having regular menstrual cycles. There was history of use of medication like diuretics, vasodilators, and β-blockers. None of the family members had any illness including cardiac disease.
Discussant: Clinical presentation of this 18 years old female, with exertional dyspnea, pedal edema, and PND attacks suggestive of decompensated heart failure (HF). The possible differential diagnoses are discussed below.
Dilated Cardiomyopathy
Most common presenting symptoms are SOB and pedal edema which are progressive in nature. Our patient presented with history of SOB and pedal edema for 6 months' duration whereas dilated cardiomyopathy (DCMP) patients usually have longstanding complaints. Risk of having supraventricular and ventricular arrhythmias1 are more in these patients. So new onset of arrhythmias may precipitate symptoms in patients who are asymptomatic previously.
Viral myocarditis: Patients present with SOB, palpitations, and often have history of prodromal symptoms and fever. This patient presented with SOB of a shorter duration with pedal edema. So, viral myocarditis should be strongly considered as a possible diagnosis. Our patient did not report any fever or prodromal symptoms.
Hypertrophic Cardiomyopathy
Patients with hypertrophic cardiomyopathy (HCM) can present at any age but mostly between third and fourth decade.2 Exertional SOB, exercise intolerance, palpitations, angina, presyncope, and syncope are common in these patients with arrhythmias, most commonly atrial fibrillation, act as triggering for HF.3 In later stages these may present as DCMP. In 60% of the patients family history of HCM is present.4 Our patient presented at 18 years of age with sudden onset symptoms, so HCM with arrhythmia can be considered.
Chronic Rheumatic Heart Disease
Most common cause of mitral stenosis (MS) is rheumatic heart disease (97.4%) and females are affected more. Isolated mitral regurgitation is seen in 41.1% of chronic rheumatic heart disease patients.5 6 Patients with MS usually present with longstanding and progressive dyspnea. MS was considered in our patient as she presented with SOB and PND episodes but the duration was less than 1 year.
Pulmonary Arterial Hypertension
Most common presenting complaint is breathlessness. Most present with dyspnea and/or fatigue, whereas edema, chest pain, and presyncope are less common and associated with advanced disease. This patient presented with dyspnea and pedal edema. Hence, pulmonary arterial hypertension (PAH) causing right HF is to be considered in this patient. But PND is not a feature of primary PAH. In thromboembolic PAH shower of emboli may mimic like PND. Secondary PAH due to left heart disease also is possible.
Myocarditis with Collagen Vascular Diseases
Patients with collagen vascular disease usually presents with joint pains, cutaneous manifestations, fever, and myalgias. They can have cardiac complaints like SOB, chest pain, and palpitations due to pericarditis, myocarditis, and valvular lesions. Most commonly cardiac symptoms occur in patients with established disease, but they can occur as an initial presentation which is rare. Our patient did not have any skin lesions, joint pains, and other systemic involvement features to suggest presence of collagen vascular disease.
Infiltrative Cardiomyopathy
Patients with infiltrative cardiomyopathy commonly present with SOB, and may complain of palpitations due to risk of arrhythmia. Common age at presentation is sixth decade as part of multisystem disorder.7 This patient presented in young age and had biventricular failure symptoms but has no complaints pertaining to other system involvement.
Endomyocardial Fibrosis
Endomyocardial fibrosis (EMF) commonly affects younger age group patients. In tropical countries it contributes for highest number of restrictive cardiomyopathy cases. This patient presented with PND attacks and pedal edema suggestive of biventricular failure. So it was considered as possible diagnosis, but in the last it was rejected because it was not common in the patient's geographical area (Telangana, India). It is common in Kerala and Tamil Nadu in South India. It accounts for 5 to 7% of all HF admissions in South India and < 5% of all HF admissions in North India.8 9
Presenter: On general physical examination, patient was conscious and coherent. Moderately built and well nourished. Body temperature was 97.4°F. Pulse rate was 76 beats per minute, regular rhythm, and normal character; blood pressure was 100/70 mm Hg in the right arm in supine position. Thoracoabdominal breathing was 18 cycles/min and SpO2 99% on ambient air. Pedal edema was present, but no pallor, icterus, cyanosis, clubbing, or lymphadenopathy. There was no skin rash or joint swelling. On cardiovascular examination, mean column height of jugular venous pressure (JVP) elevated to 10 cm H2O. Point of maximum impulse was heaving type, localized in the left 5th intercostal space along the midclavicular line. First and second heart sound normal in intensity and second heart sound normal in split with A2 > P2. No S3, S4, or murmurs heard. Normal vesicular breath sounds heard on respiratory examination. Per abdomen soft, nontender, no free fluid in the peritoneum, and no organomegaly. No focal neurological deficits found on neurological examination.
Discussant: On general physical examination, patient was afebrile, JVP elevated, S1 and S2 normal in intensity, no S3 and S4, and no murmurs heard. Apex impulse was heaving type Clinically, no evidence of PAH. No murmurs to think of primary valvular disease. No cutaneous manifestations and join involvement present.
Possible Differential Diagnosis
HCM
Patients with HCM may have failure symptoms at any age, mostly due to mitral regurgitation or diastolic dysfunction. Usually, they have bisferiens pulse, heaving type of apex with palpable S4, while patients with left ventricular (LV) outflow tract obstruction have systolic ejection murmur at apex and left lower sternal border.10 In patients without subaortic gradient systolic murmur will be present or absent. Our patient did not have any murmur or palpable S4 but had apex of LV type.
Infiltrative Cardiomyopathy
These patients usually present with HF due to diastolic dysfunction. In sarcoidosis patients present with restrictive cardiomyopathy with conduction abnormalities and bradyarrhythmias. In amyloidosis patients initially have ventricular hypertrophy but in end stages they presents as DCMP with biventricular failure and have increased risk of tachyarrhythmias.7 11 Our patient presented with symptoms of biventricular failure with no conduction abnormalities. Hence, it has to be considered although she presented at young age.
Endomyocardial Fibrosis
EMF is common in young age patients. A bimodal peak with onset in the first decade and second peak in the second to fourth decades of life. Most patients present with symptoms from left or right restrictive physiology including dyspnea on exertion, PND, pedal edema, and ascites. It is characterized by apical fibrosis of the right ventricle (RV), LV, or both ventricles and mitral, tricuspid subvalvular apparatus leading to regurgitation. In patients with EMF in South India, 20% had biventricular failure, 60% had RV failure (RVF), and 20% had LV failure.12 In this case because of PND episodes and right side failure, biventricular involvement is more likely. Then clinically we may have systolic murmur due to tricuspid or mitral regurgitation, additional sounds like S3 or S4 and mild to moderate PAH signs. All of these signs are not there.
Presenter
Investigations
-
Biochemical investigations
-
Electrocardiogram (ECG)
-
Chest X-ray
-
Echocardiogram (ECHO)
-
Other investigations
Investigations are shown in Table 1.
|
Parameters |
Patient range |
|---|---|
|
Hemoglobin (g/dL) |
13.0 |
|
White blood cells |
9,200 |
|
Platelet count |
320,000 |
|
Na+/K+ (meq) |
137/4.4 |
|
Creatinine/urea (mg/dL) |
0.78/29 |
|
Total/direct bilirubin |
0.5/0.2 |
|
AST/ALT (U/L) |
43/34 |
|
Total protein/Albumin (gm/dL) |
7.5/4.5 |
|
PT/INR/APTT |
11.4/0.97/ 26.4 |
|
Total cholesterol/Triglycerides (mg/dL) |
138/78 |
|
HDL/LDL/VLDL (mg/dL) |
38/84/16 |
|
Hs CRP (mg/L) |
0.95 |
|
NT-Pro BNP (pg/mL) |
3,649 |
|
HS Trop I (ng/L) |
12.9 |
|
CPK/LDH (u/L) |
51/243 |
Abbreviations: ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate transaminase; CPK, creatine phosphokinase; CRP, C-reactive protein; HDL, high-density lipoprotein; HS Trop I, high-sensitivity troponin I; INR, international normalized ratio; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; NT-Pro BNP, N-terminal pro-brain natriuretic peptide; PT, prothrombin time; VLDL, very low-density lipoprotein.
Note: Routine laboratory investigations as shown in the table are within normal except elevated levels of NT-Pro BNP.
In HCM there are deep T inversions. In infiltrative cardiomyopathy low voltage complex ECG with ventricular hypertrophy is common. Our patient had biventricular hypertrophy with enlargement of both atria. So, low voltages are not seen (Fig. 1).

-
Fig. 1 Combined ventricular hypertrophy. Left ventricular hypertrophy (LVH) with strain pattern and right and left atrial enlargement.
Fig. 1 Combined ventricular hypertrophy. Left ventricular hypertrophy (LVH) with strain pattern and right and left atrial enlargement.
In Fig. 2 parasternal short-axis view of transthoracic ECHO showing concentric LV hypertrophy (LVH) and speckle appearance of myocardium.
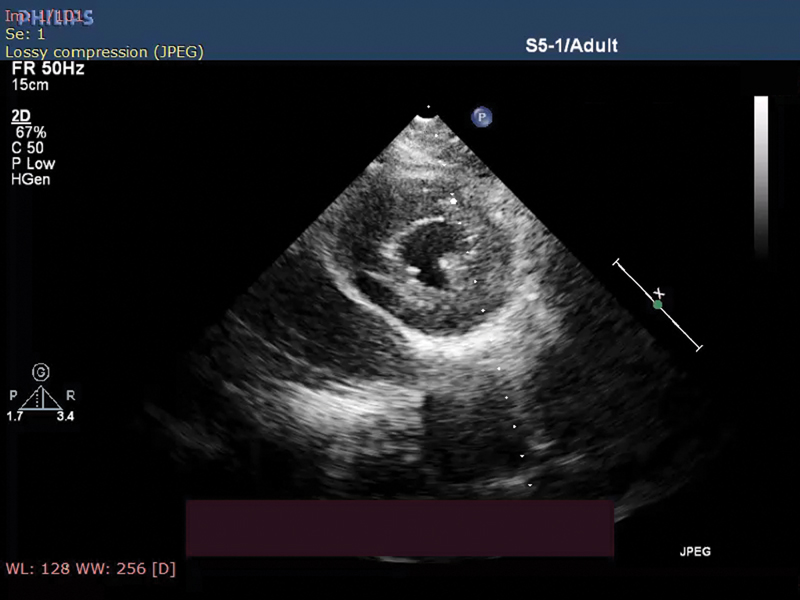
-
Fig. 2 Parasternal short-axis showing left ventricular (LV) hypertrophy with speckled pattern.
Fig. 2 Parasternal short-axis showing left ventricular (LV) hypertrophy with speckled pattern.
Fig. 3 shows the apical four-chamber view during systole showing enlargement of both atria, LVH with thickening of interatrial and interventricular septum (IVS) along with speckle appearance.

-
Fig. 3 (A) Apical four-chamber view in systole showing biatrial enlargement (left atrium [LA] > right atrium [RA]), hypertrophic left ventricular (LV), speckled pattern in interventricular septum, and thickening of interatrial septum. (B) Apical four-camber view in diastole.
Fig. 3 (A) Apical four-chamber view in systole showing biatrial enlargement (left atrium [LA] > right atrium [RA]), hypertrophic left ventricular (LV), speckled pattern in interventricular septum, and thickening of interatrial septum. (B) Apical four-camber view in diastole.
Apical four-camber view in diastole showing features of thickened IVS and speckle pattern of myocardium.
Discussant
ECHO suggestive of biatrial dilatation with more dilated left atrium (LA), wall thickening of both ventricles, and interatrial septum (IAS). Speckled pattern is seen in IVS.
Echocardiographic mild regurgitations of both or either of atrioventricular (AV) valve if present then possibility to consider is biventricular involvement of EMF. But classical feature of EMF is apical obliteration which was not seen in this condition. Mild to moderate PAH may there. Pulse Doppler velocity of mitral valve will show restrictive pattern. Low tissue Doppler velocities of AV valves and myocardium are the features of this condition. Asymmetric septal hypertrophy and systolic anterior motion of mitral valve with mitral regurgitation are features of HCM, which were not seen in this patient. Concentric hypertrophy of LV along with right ventricular hypertrophy (RVH) and interatrial septal thickening features suggestive of amyloidosis.
No valvular regurgitation and evidence of PAH seen on color Doppler and velocity of propagation was 40 cm/s. Pulse Doppler was suggestive of grade 1 diastolic dysfunction. On tissue Doppler imaging, lateral mitral annulus velocities [e'] reduced, E/e' ratio 5 with deceleration time 100 ms suggestive of diastolic dysfunction.
In Fig. 4, tissue Doppler shows reduced e' with E/e ratio of 5 and decreased deceleration time features suggestive of diastolic dysfunction.

-
Fig. 4 Tissue Doppler imaging showing reduced lateral mitral annulus velocities.
Fig. 4 Tissue Doppler imaging showing reduced lateral mitral annulus velocities.
Possibilities based on Two-Dimensional ECHO Findings
Amyloidosis
Characteristically amyloid patients will have the speckle pattern in addition with thickening of IAS. These changes are due to the deposition of amyloid protein into the tissue.13 14 These patients usually present with biventricular failure symptoms with elevated E/E' ratio.15 Our patient had classical features of amyloidosis with biventricular hypertrophy, thickened IAS, and sparkling appearance of the myocardium. Our patient presented with biventricular failure symptoms with moderate LV systolic and grade 1 diastolic dysfunction. These findings make amyloidosis a strong possibility in this case.
Sarcoidosis
Classical presentation of sarcoidosis is restrictive cardiomyopathy but patients may present as DCMP.16 17 These patients usually have hematological features like anemia, leucopenia, and lymphopenia with eosinophilia but our patient does not have any of these to support the diagnosis of sarcoidosis. But the duration of symptoms will be more in these patients. Sarcoidosis patients usually show anemia with leucopenia and lymphopenia with eosinophilia which were not seen in this patient. Our patient presented with symptoms less than 1-year duration and she did not have any symptoms related to upper or lower respiratory tract involvement.
Discussant
Based on the above findings amyloidosis is likely possible diagnosis in this patient but there was no ECG-ECHO discrepancy, with biventricular involvement and dominant RVF with RV dysfunction EMF cannot be ruled out.
To distinguish the different causes of infiltrative cardiomyopathy and to rule out other causes of restrictive cardiomyopathy, cardiac magnetic resonance imaging (MRI) was advised.
Radiologist opinion: Cardiac MRI suggestive of concentric LVH with end diastolic wall thickness EDWT in septum 15 mm and RVH. LA dilated more than right atrium, thickened IAS, and diffuse subendocardial late gadolinium enhancement of LV myocardium seen. Transmural enhancement is seen in the septum in mid one-third, inferior and lateral walls in apical third, and apex of LV. These features are suggestive of cardiac amyloidosis.
Cardiac MRI (Figs. 5 and 6) suggestive of concentric LVH, dilated LA with thickening of both IVS and IAS. Diffuse subendocardial late gadolinium enhancement seen after giving contrast features suggestive of amyloidosis.

-
Fig. 5 Cardiac magnetic resonance imaging (MRI): (A) Cardiac MRI short-axis view showing concentric left ventricular (LV) hypertrophy. (B) Four-chamber view showing interventricular septum (IVS) thickening. (C) Four-chamber view showing interatrial septum (IAS) thickening. (D) Dilated left atrium.
Fig. 5 Cardiac magnetic resonance imaging (MRI): (A) Cardiac MRI short-axis view showing concentric left ventricular (LV) hypertrophy. (B) Four-chamber view showing interventricular septum (IVS) thickening. (C) Four-chamber view showing interatrial septum (IAS) thickening. (D) Dilated left atrium.

-
Fig. 6 Postcontrast images showing diffuse subendocardial enhancement.
Fig. 6 Postcontrast images showing diffuse subendocardial enhancement.
Discussant
Sarcoidosis
Classical features of sarcoidosis on cardiac MRI due to myocardial fibrosis not confined to any specific territory with involvement of basal and mid ventricular septum and transmural involvement will not be there.18 But in our case there is transmural involvement of the septum in mid one-third, inferior and lateral walls in apical third, and apex of LV.
Amyloidosis
Classical features of cardiac amyloidosis is septal thickening with subendocardial late gadolinium uptake and both ventricular hypertrophy involving atria also due to deposition of amyloid into the tissues.19 Our patient had all these classical features like biventricular hypertrophy, thickened IVS and IAS, diffuse subendocardial late gadolinium enhancement of LV myocardium, and transmural enhancement seen in the septum in mid one-third, inferior and lateral walls in apical third, and apex of LV. These findings are suggestive of cardiac amyloidosis. Cardiac MRI cannot differentiate the types of amyloidosis.
To differentiate different types of cardiac amyloidosis other tests are required to support each of them like ultrasound abdomen, protein electrophoresis with immunofixation, and kappa and lambda free light chain levels and ratio. Also, investigations like serum calcium and angiotensin-converting enzyme (ACE) levels to rule out sarcoidosis.
Presenter
Amyloidosis
To rule out the systemic amyloidosis both urine and serum protein electrophoresis in combination with immunofixation is helpful. These are positive only in 20 to 40% of the transthyretin type of amyloidosis.19 In our patient, both serum and urine protein electrophoresis with immunofixation were negative and kappa and lambda free light chain ratio were normal.
Sarcoidosis
Hypercalcemia is reported in 63% of patients with sarcoidosis.20 Our patient had normal serum calcium. Serum ACE levels are elevated in 60% patients with systemic sarcoidosis and 21% patients with cardiac sarcoidosis.21 This patient had normal serum ACE levels. These patients may have raised inflammatory markers, which were normal in our patient.
There is no abnormality in the form of hepatosplenomegaly on ultrasound and no lymphadenopathy seen on computed tomography chest in this patient.
Table 2 shows negative results for systemic amyloidosis with normal ACE, calcium levels, and free light chains.
|
Parameter |
Patient range |
|---|---|
|
S. angiotensin-converting enzyme (ACE) levels (uL) |
19 |
|
Serum calcium (mg/dl) |
8.6 |
|
Complete urine analysis |
Normal |
|
Serum protein electrophoresis (SPEP) |
Negative |
|
Urine protein electrophoresis (UPEP) |
Negative |
|
Serum kappa/lambda FLC ratio |
1.10 |
|
RF IgM ELISA |
Negative |
|
Kappa light chain/lambda light chain |
35.45/32.15 |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; FLC, free light chain; IgM, immunoglobulin M; RF, rheumatoid factor.
Discussant
Specific laboratory investigations rule out the amyloid light-chain type amyloidosis; no other systemic involvement suggestive of amyloidosis and sarcoidosis.
There was no extra cardiac evidence suggestive of sarcoidosis or amyloidosis in this case. Previously, endomyocardial biopsy (EMB) was indicated to differentiate the different types of infiltrative cardiomyopathies. EMB remains the gold standard for transthyretin type of amyloidosis (transthyretin cardiac amyloidosis [ATTR-CA]), it has 100% sensitivity and specificity of biopsy specimens collected from four or more sites. Congo red staining is useful for identification of amyloid deposits.22 But EMB is an invasive procedure with procedure-related complications. Only alternative for invasive cardiac biopsy to diagnose ATTR-CA with accuracy is nuclear scintigraphy using bone avid radiotracers. An international collaboration with large cohort of EMB-proven cases of ATTR-CA concluded that these bone avid tracers are 100% specific when grade 2 or 3 uptake is seen in the absence of monoclonal gammopathy and serum urine protein electrophoresis. These nuclear scans are noninvasive and easier but expensive compared with EMB. Technetium 99m pyrophosphate (PYP) is a radiotracer used for bone scintigraphy. This scan has recently emerged as a noninvasive diagnostic test that is 92% sensitive and 95% specific for ATTR-CA in the absence of monoclonal gammopathy.19 One to 3 hours after injection, single-photon emission computerized tomography images can be interpreted by both semiquantitative and quantitative methods. In semiquantitative method grade > 2 and in quantitative method it depends on the uptake of tracer surrounding the heart, value of > 1.5 is suggestive of amyloidosis. In our patient, semiquantitative method has grade 2 value and quantitative method has heart to contralateral (H/CL) ratio > 1.5 which were strongly suggestive of transthyretin type of cardiac amyloidosis.23
Presenter
In this patient, PYP scan showed grade 2 radiotracer uptake with H/CL ratio of 1.6, which is strongly suggestive for scintigraphically detectable transthyretin type of amyloidosis (Fig. 7).

-
Fig. 7 Technetium 99m pyrophosphate (PYP) scan.
Fig. 7 Technetium 99m pyrophosphate (PYP) scan.
Fig. 7 shows increased uptake of radiotracer in the heart compared with contralateral lung region with H/CL ratio of 1.6 suggestive of ATTR-CA.
Discussant
In our patient confirmation of amyloidosis is by negative urine and serum electrophoresis and positive Tc 99m PYP scan which is characteristic of ATTR-CA and has sensitivity of 92%, specificity of 95%, and 100% positive predictive value. Positivity by nuclear scan is due to deposition of mutant form of amyloid protein which is specific for ATTR-CA. Transthyretin amyloidosis is of two types, wild and mutant type. Wild-type transthyretin amyloidosis is more common than mutant type and males are affected more, usually after 70 years of age whereas mutant type is common in younger age group.24 25 Nervous system involvement is common in ATTR-CA with bilateral carpel tunnel syndrome being the most common. Our patient did not have any neurological complaints.
Clinical Diagnosis
Transthyretin Amyloid Cardiomyopathy
Discussion of Management
Depending on the levels of troponin T and N-terminal pro-brain natriuretic peptide, patients of transthyretin-type amyloidosis are classified into three stages with both, one, or none of the markers below threshold as stage I, II, and III, respectively, with median survival of 66, 40, and 20 months.25 In our patient both markers were elevated and hence classified as stage III disease.
According to newer study based on estimated glomerular filtration rate (eGFR), transthyretin amyloid patients are classified into three stages with median survival of 69, 47, and 24 months in stages I, II, and III. Patients with wild-type ATTR have longer survival than mutant type ATTR.26 Based on this study our patient had stage I illness with eGFR OF 111 mL/min/m2.
During hospitalization the patient was treated with diuretics, ACE inhibitors, and β-blockers. Tafamidis is a transthyretin stabilizer, advised to the patient as it reduces all-cause mortality, recurrent hospitalizations, and improves the quality of life by reducing the decline in functional capacity.
Final Diagnosis
Transthyretin amyloid cardiomyopathy.
Conflict of Interest
None declared.
References
- Cardiomyopathy. Diagnosis and management of dilated cardiomyopathy. Heart. 2000;84(01):106-112.
- [Google Scholar]
- New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254.
- [Google Scholar]
- Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517-2524.
- [Google Scholar]
- Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(07):749-770.
- [Google Scholar]
- Surgical pathology of the mitral valve: a study of 712 cases spanning 21 years. Mayo Clin Proc. 1987;62(01):22-34.
- [Google Scholar]
- Pathology of surgically excised mitral valves. One hundred consecutive cases. Arch Pathol Lab Med. 1985;109(09):823-828.
- [Google Scholar]
- Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101(21):2490-2496.
- [Google Scholar]
- Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232-2239.
- [Google Scholar]
- Infiltrative cardiovascular diseases: cardiomyopathies that look alike. J Am Coll Cardiol. 2010;55(17):1769-1779.
- [Google Scholar]
- Left ventricular endomyocardial fibrosis in India. Br Heart J. 1977;39(05):563-568.
- [Google Scholar]
- Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging. 2017;10(04):398-407.
- [Google Scholar]
- Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PLoS One. 2014;9(12):e115910.
- [Google Scholar]
- Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14(03):205-212.
- [Google Scholar]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(02):736-755.
- [Google Scholar]
- Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(07):624-632.
- [Google Scholar]
- Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11(01):e007030.
- [Google Scholar]
- Cardiac amyloidosis: overlooked, underappreciated, and treatable. Annu Rev Med. 2020;71:203-219.
- [Google Scholar]
- Sarcoidosis in India: a review of 125 biopsy-proven cases from eastern India. Sarcoidosis. 1990;7(01):43-49.
- [Google Scholar]
- Sarcoidosis: an Indian perspective.Postgraduate Medicine. Bombay: Association of Physicians of India; 1998. p. :472-480. In: ed.
- [Google Scholar]
- Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med. 1988;148(03):662-666.
- [Google Scholar]
- Standardization of 99mTechnetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol. 2018;25(01):181-190.
- [Google Scholar]
- Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(02):e000098.
- [Google Scholar]
- Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014-1020.
- [Google Scholar]
- A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799-2806.
- [Google Scholar]






