Translate this page into:
Substrate Modification of Ischemic Scar in the Left Ventricle
*Corresponding author: Atul Kaushik, Department of Electrophysiology, Fortis Escorts Heart Institute, New Delhi, India. dratulkaushik0126@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaushik A, Jaswal A. Substrate Modification of Ischemic Scar in the Left Ventricle. Indian J Cardiovasc Dis Women. 2024;9:191-2. doi: 10.25259/IJCDW_25_2024
An 85-year-old patient, known case of hypertension, diabetes mellitus, hypothyroidism, coronary artery disease, P/percutaneous transluminal coronary angioplasty, P/coronary artery bypass grafting, P/cardiac resynchronization therapy-D, heart failure with reduced ejection fraction (HFrEF), left ventricular (LV) ejection fraction = 30%, and paroxysmal atrial fibrillation, presented with two episodes of syncope in the emergency department. On device evaluation, the patient was found to have received appropriate therapies for ventricular tachycardia (VT)/ventricular fibrillation (VF) episodes despite of optimal medical therapy with metoprolol succinate extended release, amiodarone, and mexiletine. The patient was admitted and laboratory records were unremarkable except for raised N-terminal pro–b-type natriuretic peptide (NT-ProBNP) level of 10054 pg/mL. Following decongestive therapy, the patient was planned for VT substrate mapping using CARTO 3D mapping system. Echocardiography was suggestive of regional wall motion abnormalities and it ruled out LV apical clot. VT induction was not attempted as patient had previous episodes of hemodynamic unstable VT. The cutoff value bipolar voltage was set at 0.5 mV for densely scarred area. Scar homogenization was performed at sites with fractionated potentials and late potentials, as shown in Figure 1. VT was non-inducible with ventricular extrastimulations. The patient underwent procedure without any complication and was discharged after 2 days. The patient was asymptomatic and VT/VF free on 24 h Holter at follow-up at 2-, 6-, and 8-weeks post-procedure. Furthermore, NTProBNP also decreased to a level 800 pg/mL at follow-up.
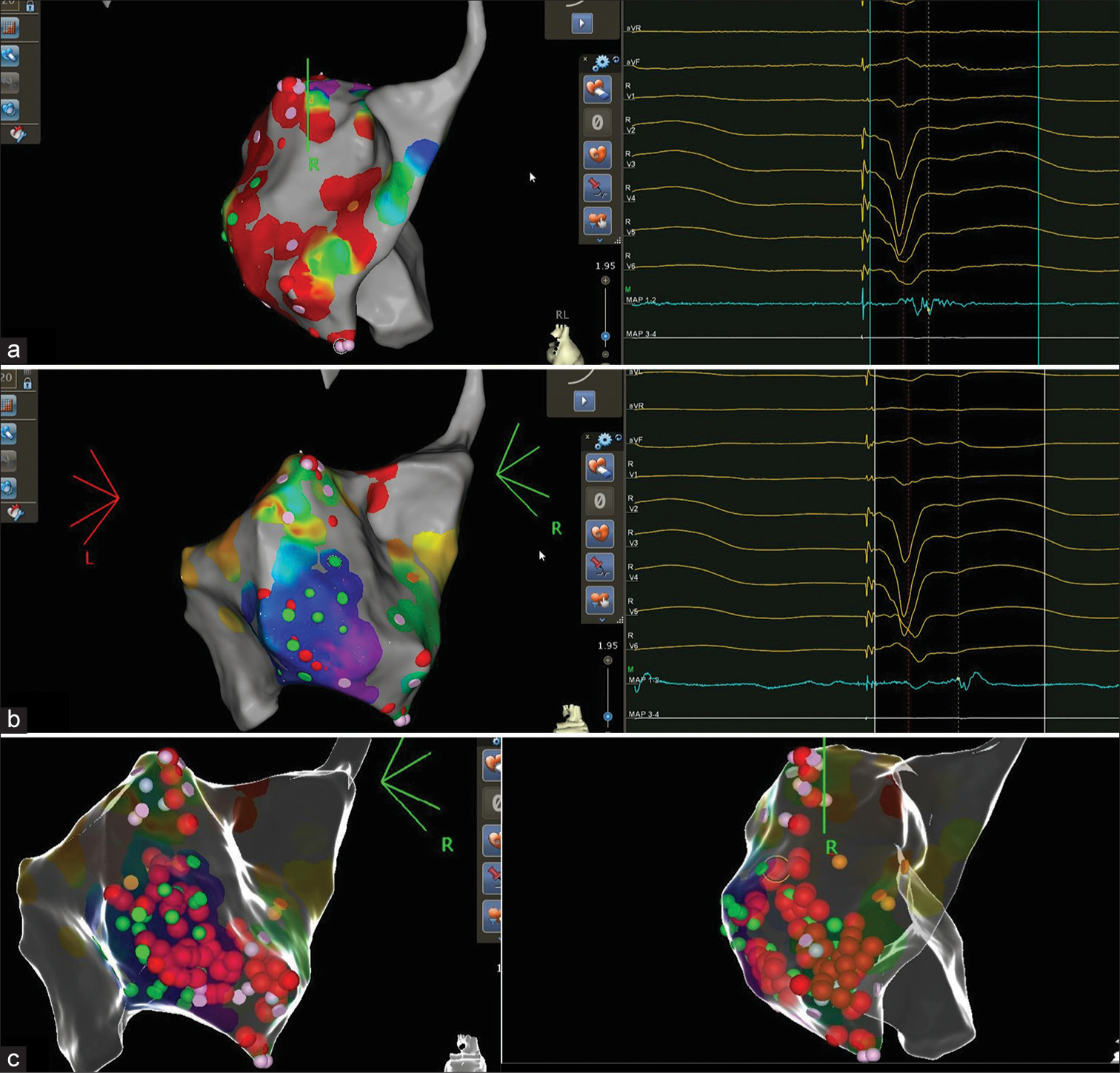
- (a) Fractionated potentials. (b) Late potentials. (c) Ablation tags.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.






