Translate this page into:
Practical Approach to Diagnosis, Prevention, and Management of Coronary No-Reflow
*Corresponding author: Lalita Nemani, Consultant cardiologist, Department of Cardiac Sciences, Dr. Ismail Surgical Center, Dubai, United Arab Emirates. drlalita775@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nemani L. Practical Approach to Diagnosis, Prevention, and Management of Coronary No-Reflow. Indian J Cardiovasc Dis Women 2023;8:65-74.
Abstract
Coronary no-reflow (NR) defined as inadequate myocardial perfusion despite restoration of coronary artery patency is a bane for an interventional cardiologist. It can complicate percutaneous coronary interventions especially in the setting of STEMI and dampens the potential benefits of PPCI. Broadly classified as Reperfusion NR and Interventional NR, mechanism is multifactorial. The basic underlying culprit is microvascular obstruction either secondary to distal embolization, intravascular plugging, or ischemic reperfusion injury. Coronary angiogram is an easy, readily available, and essential modality to diagnose no-reflow, but the gold standard is gadolinium-enhanced cardiovascular magnetic resonance imaging. Preventive strategies for NR should be integral part of prePCI planning especially in clinical scenario where NR is expected such as STEMI with delayed presentation and high thrombus burden, atherectomy, and SVG PCI. The cornerstone of treatment for NR is local vasodilators and antiplatelet therapy to ameliorate vasospasm and thromboembolism respectively, and different combinations of the two should be used in no specific order to achieve reversal of NR. NR phenomenon is associated with poor short-term and long-term prognosis and every attempt should be made to avoid or reverse it. Therapeutic hypothermia, hyperoxemic reperfusion therapy, targeted anti-inflammatory approach, and cellular approach appear proising but further research is mandatory.
Keywords
No-reflow
Coronary imaging
Therapeutic hypothermia
Pharmacological vasodilation
Antiplatelet therapy
INTRODUCTION
Coronary no-reflow (NR) is defined as inadequate myocardial perfusion despite restoration of coronary artery patency in the absence of dissection or spasm.[1] Angiographically, NR is thrombolysis in myocardial infarction (TIMI) flow Grade <3 and myocardial blush Grade (MBG) <3 manifesting as reduced or absent flow in the affected coronary vessel despite removal of primary obstruction.[2] TIMI flow Grade 0–1 is referred as NR, and TIMI flow grade as slow flow. The different terms no flow/NR/low flow/slow flow/slow reflow apparently are expressions for the same abnormality and simply referred as NR.
NR complicates nearly 50% cases of STEMI, less frequent in NSTEMI. Incidence in elective cases is <1.5% with a higher occurrence of nearly 4% in saphenous venous graft interventions.[3]
CLASSIFICATION
NR is broadly classified as reperfusion NR and interventional NR [Figure 1].
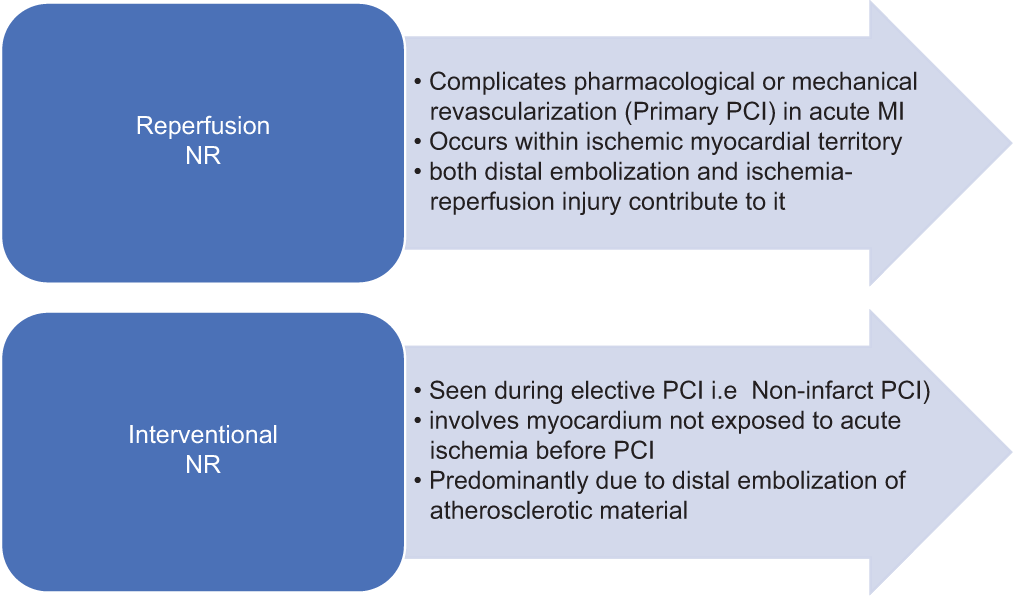
- Classification of no-reflow (NR).
PATHOGENESIS
The mechanism of NR is multifactorial [Figure 2]. The basic underlying culprit is microvascular obstruction (MVO) secondary to distal embolization, intravascular plugging, or ischemic-reperfusion injury.[4-6]
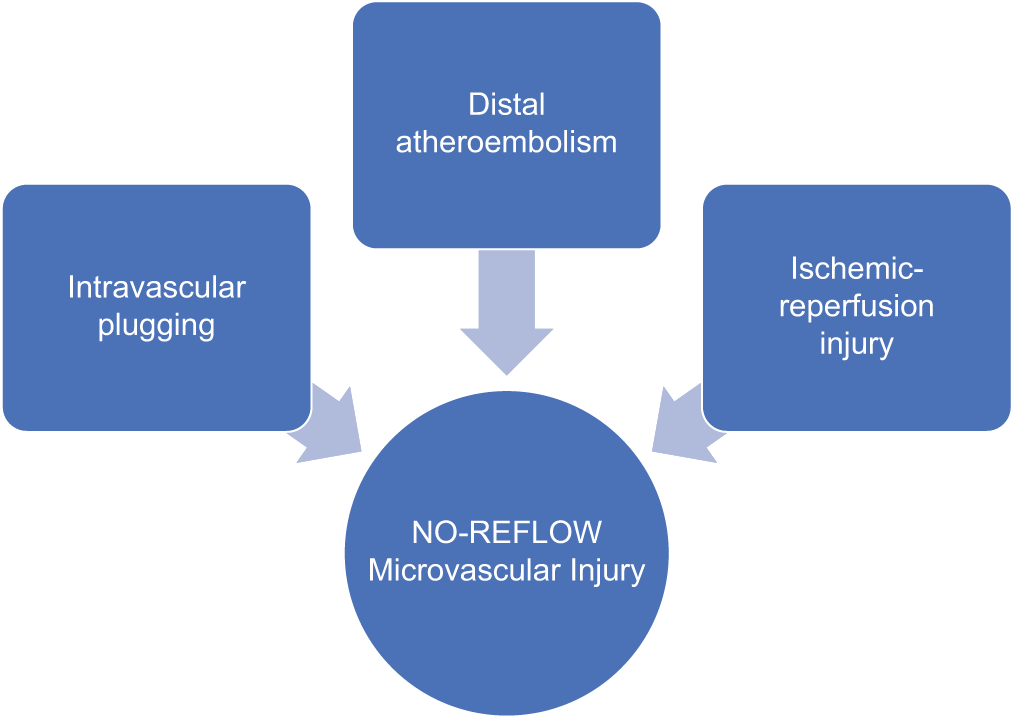
- Pathogenesis of no-reflow.
CLINICAL PRESENTATION
Clinical scenario varies from asymptomatic state to hemodynamic compromise with life threatening arrhythmias. Reperfusion NR may be asymptomatic or present as ongoing chest pain and non-resolution of ST-segment elevation. Interventional NR classically with acute chest pain and ECG changes of ischemia which may over time. Acute ischemia presents as hemodynamic compromise prompting early initiation of strategies to reverse NR. Even in asymptomatic cases, every attempt should be made to correct NR, as it has poor outcome both short- and long-term.
PREDICTORS FOR NR
Certain clinical and angiographic factors predispose to Noreflow [Table 1].
| Predictors of individual susceptibility | Predictors of ischemic reperfusion injury | Angiographic predictors |
|---|---|---|
| • Age >65 • Female sex • Hypertension • Hyperglycemia • Smoking • Dyslipedemia • Atrial fibrillation • Renal insufficiency • Genetic- adenosine 2A receptor polymorphism (1976T>C)/ lysis resistance |
• Time to treatmentdelayed presentation (>6 hours) • Larger area of infarction (LAD occlusion) • Mean platelet volume • Oxidative stress • Thromboxane A2 levels • Vasoconstrictor ET-1 levels • Inflammatory markersneutrophil count, CRP |
• High thrombus burden, • Plaque composition • SVG PCI, • Long lesions (> 15 mm) |
RISK SCORES FOR NR
Wang risk score[7] based on seven clinical parameters and Yip et al.[8] angiographic score help to predict NR.
Platelet lymphocyte ratio at admission is a strong predictor of NR (Topark et al.) [Table 2].[9-30]
| MELD SCORE [Model for end-stage liver disease-XI score] | MELD-XI score = 5.11×(total bilirubin, mg/dL) +11.76× (creatinine, mg/dL) +9.44 Bilirubin: Potent endogenous antioxidant, suppresses ROS production,[10]inhibits oxidative stress that contribute to no-reflow.[11] High bilirubin levels-NR(Celik et al.[12]) Creatinine: Marker of renal impairment. Independent marker for NR in STEMI patients undergoing PCI. NR in renal dysfunction attributed to ROS and endothelial dysfunction |
• Independent predictor of NR during Primary PCI • Highly predictive power for no-reflow • May be useful in early risk stratification of patients with STEMI[15,16] |
| • Both renal insufficiency and NR are manifestations of oxidative stress[13,14] | ||
| R2-CHA2DS2-VAsc score [Renal function, Congestive heart failure, Hypertension, Diabetes, Age, peripheral Vascular disease, Stroke and female Sex |
1 point each 2 for Stroke and Age ≥75 years 2 points for renal function (eGFR) ≤ 60 mL/min/1.73 m2 using the MDRD formula) |
• High CHA2DS2-VASc score (≥3)-independent predictor of no-reflow (80.9% sensitivity and 74.6% specificity).[19] • The R2-CHA2DS2-VASc score-relatively poor predictive value for no-reflow. Score ≥3–52.6% sensitivity and 73.1% specificity for no-reflow.[20] |
| Novel Biomarker: Malat1 [Metastasis-Associated Lung Adeno-Carcinoma Transcript 1] Long Non-Coding Rnas (Lnc Rnas)[21]Are Transcripts With ≥200 Nucleotides. Recognised For Their Role In Many Biological Processes (Malat1)[22]Conserved Lnc Rna, Biomarker In Lung Malignancy. Recently Identified For Its Role In Regulation Of Many Pathophysiological Processes Related To Vascular Disease |
Mi RNAs namely miR-30e and miR-126[23,24]biomarkers for predicting NR in STEMI patients Elevated HPSE and EDN-1[25-27]are associated with no-reflow in STEMI |
• MALAT1 regulates the expression of miR-155 • Expression of MALAT1 is significantly increased and that of miR-30e, miR-126 and miR-155 suppressed in the patients with NR |
Expression of C-reactive protein (CRP), Heparanase (HPSE) and (Endothelin-1) EDN1 is significantly up-regulated in patients with no-reflow.[28] CRP causes microvasculature obstruction by complementary activation and neutrophil plugging and contributes to NRP. No-reflow is associated with higher values of CRP after PCI (12.10 vs. 8.13 with a P = 0.017)[16-18]
DIAGNOSIS OF NR
Electrocardiograph
Resolution of ST elevation following revascularization is highly specific (91%) for reestablishment of myocardial perfusion but less sensitive (77%).[29,30] ST resolution should be more than 70% at 60 min after PCI, anything less is a sign of NR.
Biochemical markers
Serial measures of cardiac biomarkers, namely, troponins, creatinine kinase MB, and serum myoglobin are useful for the assessment of patency in the infarct artery.[31] They are estimated at baseline and then either 60 or 90 min after completion of reperfusion therapy. Greater increase in these markers over time from baseline indicates successful reopening of the occluded epicardial vessel, and also establishment of micro vascular tissue reperfusion.
Myocardial contrast echo
A bedside procedure to demonstrate NR where micro bubbles of an ultrasonic contrast agent is injecting intravenously. Absence of opacification of the myocardium demonstrates NR.[32,33]
Coronary angiogram
Easy, readily available, and essential modality of diagnosing NR in the catheterization laboratory.
TIMI flow grade – Only TIMI 3 flow indicates successful reperfusion, a flow <3 is considered as NR.[34]
MBG – MBG allows further risk stratification in patients with TIMI 3 flow, with a MBG score of 0–1 indicates NR.[35]
Corrected TIMI frame count – Another method to evaluate epicardial flow. Low frame counts after PPCI indicates favorable reperfusion.[36]
Myocardial perfusion grade (TMPG)-In TIMI 3 flow, TMPG is a technique to assess myocardial perfusion or “blush” on a coronary angiogram. A TMPG score of 0/1 indicates impairment of microvascular flow, whereas a TMPG grade 2/3 implies salvaged myocardium.
Intracoronary doppler
Although coronary angiogram is the most frequently done procedure for NR, it is not accurate. A measure of flow parameters and resistance parameters is a more accurate invasive technique for diagnosis of NR.
Coronary flow reserve (CFR)
A CFR value <2.0 indicates presence of MVO with nearly 80% sensitivity. NR is characterized by typical flow pattern of systolic flow reversal (retrograde) and rapid diastolic flow deceleration.
Microvascular resistance index (IMR)
IMR = distal coronary pressure X mean transit time of a bolus at maximum hyperemia. It is an independent measure of microcirculatory flow
Angiography-derived IMR (IMR Angio)
Latest addition to the armamentarium. IMR angio can predict and IMR more than 40 units and the presence of large MVOs on cardiac magnetic resonance imaging (MRI) with good accuracy.[41]
Corflow therapyTM
This new technology combines real-time microvascular assessment with the ability to administer intracoronary drugs.
The procedure involves transient balloon occlusion of the coronary, infusion of a crystalloid at fixed incremental dose rates, and simultaneous measurement of distal and proximal pressures
Microvascular resistance is derived from the flow and pressure quotient. The procedure is still experimental with initial encouraging results.[42]
Cardiac MRI
The gold standard[43] for NR diagnosis is gadolinium-enhanced cardiovascular magnetic resonance imaging.
MVO appears as dark hypointense areas surrounded by hyperintense necrotic myocardium on T-weighted images in delayed gadolinium enhancement images (10–15 min),[44] or as low signal images in early (1–3 min) images[45]
First-pass perfusion (FPP) method: FPP is another technique which is also contrast dependent. It can detect even small areas of MVO. However, it is not as specific as DGE. A perfusion defect appears as absence of contract-enhancement in the affected myocardium.[46]
Positron emission tomography (cardiac PET) and (SPECT) single-photon emission computed tomography for detection of NR have been described in experimental models, but not implemented practically.[47,48]
PREVENTION OF NR
NR is a challenging complication following PCI. Multiple therapies have been suggested, though none is 100% effective and doubtful impact on associated adverse cardiovascular outcome [Table 3].[49,50] As there are no ideal guidelines for the management of NR, first and foremost effort should be to prevent NR.
| Reducing the time to reperfusion from the onset of symptoms is the foremost method to salvage the myocardium and reduce no-reflow | |
|
A. Pharmacological therapy 1. Beta-blockers (Metoprolol, Carvedilol and Nebivolol) 2. ACE-inhibitors and ARBS |
Early use has shown to protect coronary microcirculation and reduce NR due to its anti-inflammatory role in pre-clinical studies Favorable results have been observed especially with fosinopril and valsartan. Guidelines recommend early initiation of these drugs in acute MI unless contraindicated |
| 3. Statins | High dose before PCI improved angiographic MVO in the STATIN STEMI.[49] Nearly 50% of reduction in CV events at 30 days was observed in the SECURE-PCI[50] study. Studies showed that patient who were already on statins at index event experienced lesser rates of NR |
|
B. Procedural tips to reduce No-reflow in the Cath lab |
1. Direct stenting and avoidance of high-pressure post-dilation especially in STEMI. 2. Short burr runs of <20 s; low burr speed of 140,000–150,000 rpm), and avoidance of decelerations more than 5000 rpm during atherectomy 3. Use of distal protection device (Percusurge and filter wire Ex device) have been proven to reduce no-reflow during elective SVG angioplasty 4. Deferred stenting in selected cases with high thrombus burden during which patient receives supportive treatment 5. Ischemic preconditioning pre and post though theoretically favor reduction of no-reflow, have not been proven so in randomized trials |
MVO: Microvascular obstruction
Strategies for prevention of NR should be incorporated in pre-PCI planning especially in clinical scenario where NR is expected such as STEMI with delayed presentation and high thrombus burden, atherectomy, and SVG PCI.
TREATMENT OF NR
The cornerstone of treatment for NR is local vasodilators and antiplatelet therapy to ameliorate vasospasm and thromboembolism, respectively. Intracoronary vasodilator and GP IIb/IIIa inhibitor are Class IIA recommendations for NR according to the 2021 ACC/AHA PCI guidelines.
Pharmacological vasodilatation [Table 4]
When sudden cessation of flow is seen in target artery during PCI; dissection, acute stent thrombosis, plaque prolapse, and vasospasm should be excluded ideally with imaging if the clinical scenario permits. If NR is confirmed by the presence of patent vessel, intracoronary vasodilators should be administered liberally ensuring an optimal activated clotting time and mechanical hemodynamic support if needed.
| MEDICATION | DOSE | MECHANISM OF ACTION | ADVANTAGE/DISADVANTAGE | EVIDENCE |
|---|---|---|---|---|
| Adenosine | 50–200 µg bolus IC 70 µg/kg/min IV |
- Dilatation of coronary microvasculature (A2 receptors mediated Smooth muscle relaxation) -Anti-inflammation - Platelet Inhibition - Ischemic preconditioning -Angiogenesis and Anti-apoptotic |
Side effects-Atrioventricular blocks, Hypotension, Dyspnoea, Bronchospasm and flushing Advantage-very short half-life (<8 s) |
REOPEN-AMI: MVO improved, peak Troponin levels reduced. Reduction in major CV events. Favourable LV remodelling at 1 year |
| Calcium channel blockers: 1. Verapamil 2. Diltiazem 3. Nicardipine |
100–250 µg bolus IC 400 µg bolus IC 50–200 µg bolus IC |
Block L-type calcium channels - Smooth muscle relaxation - Coronary - Reduce myocardial oxygen demand - Minimize damage mediated by oxygen free radicals |
Well tolerated. Effective in the treatment of NR |
Randomized trials and RECOVER-AM trial-CCB’s effective in prevention and treatment of NR, doubtful effect on improvement in outcome |
| Epinephrine | 50–200 µg bolus IC | inotropic and chronotropic properties - Alpha vasoconstriction corrects hypotension and improves coronary perfusion - Coronary vasodilation (Beta-2 receptor agonist) |
Arrhythmia - MC was sinus tachycardia Occasional SVT and non-sustained VT More common with guide catheter directed therapy than local delivery of medication |
According to Skelding et al.; both TIMI flow and TIMI frame count improved. MACCE at 30 days and 1 year improved (Darwish et al.) |
| Nitroprusside | 50–200 µg bolus IC | Nitric oxide donor - Vasodilatation, - Antiplatelet -Anti-inflammatory Properties |
Systemic hypotension | Inferior to CCB’s and Adenosine |
| Nicorandil | Bolus of 2 mg IC Followed by infusion of 8 mg/h |
Activation of ATP-sensitive potassium channels and nitric oxide donor - Vasodilatation - Neutrophil activation - Inhibition of ROS |
No randomized trials, only small studies-proved effective in NR | |
| Nitroglycerine (NTG): It is primarily a venodilator and minimal effect on arterial tone. Must be metabolized to nitric oxide to be effective which the microvascular arterioles are incapable to. Intracoronary NTG failed to show benefit in no-reflow | ||||
|
Adenosine regimen: 6 mg (One ampule or 2 mL) added to 10 cc of saline, further diluted 10-fold (add 1 cc of the solution to 10 cc saline); second dilution (again 1 cc of the solution to another 10-cc saline) to achieve final concentration of 60 µg/10 mL. To be given intracoronary injection, dose may be repeated to a maximum of 120 µg Epinephrine regimen: 1 mg (One ampule or 1 mL) added to 10 cc saline, then diluted 10-fold (add 1 cc of solution to 10 cc saline) to achieve final concentration of 10 µg/mL. 100 µg is injected intracoronary over 5 min. Dose may be repeated up to 3 times to reach a maximum dose of 400 µg |
||||
MVO: Micro vascular obstruction, TIMI: Thrombolysis in myocardial infarction
Intracoronary administration of these agents using a microcatheter or over-the-wire balloon ensure safe and effective delivery to the microcirculation with minimal adverse effects. A dedicated dual-lumen or thrombectomy catheter can also serve the purposes without losing wire position. An aspiration catheter or a pierced balloon inflated at the culprit lesion is other easily available options.
Multiple boluses of medications as mentioned above can be tried if NR persists and patient is hemodynamically stable. Different vasodilators should be tried if one does not work.
Antiplatelet therapy
The PLEIO study[51] demonstrated ticagrelor to be superior to clopidogrel in restoration of coronary microcirculation and reducing NR, also shown in a meta-analysis by Dai et al.[52] However, no difference was observed in sub analysis of PLATO trial, ATLANTIC trial, or REDUCE-MVI trial. Trial Platelet Inhibition to Target Reperfusion Injury[53] trial is an ongoing trial of Cardiac MRI too evaluate the efficacy of pre-procedural Cangrelor to reduce MVO and size of myocardial infarction.
Glycoprotein IIB/IIIA inhibitors
Potent antiplatelet agents appear promising for prevention and treatment of NR [Table 5]. However, none of the randomized trials have shown their benefit in NR. The latest ESC guidelines recommend them for use in NR or thrombotic complication (Class IIa, C).
| MEDICATION | DOSE | EVIDENCE |
|---|---|---|
| Abciximab | Bolus of 0.25 mg/kg iv followed by infusion of 0.125 µg/kg/min (max 10 µg/min) for 12 h | IC administration reduces NR and infarct size-CICERO trial and INFUSE-AMI No benefit in terms of death, re-infarction or heart failure-AIDA-STEMI |
| Eptifibatide | Bolus of 180 µg/kg, repeat 180 µg/kg bolus after 10 min, followed by infusion of 2 µg/kg/min for 18 h. If CrCl < 50 mL/min, reduce infusion by 50% |
TIMI flow grading improvement in PROTECT TIMI trial |
| Tirofiban | 25 µg/kg iv stat over 3 min, followed by infusion of 0.15 µg/kg/min for 18 h. If CrCl < 30 mL/min, reduce infusion by 50% |
Prehospital intravenous infusion achieved better STR and MBG following PCI-ON TIME-2 trial |
CICERO trial-(Comparison of Intracoronary Versus Intravenous Abciximab Administration During Emergency Reperfusion of ST-Segment Elevation Myocardial Infarction), INFUSE-AMI study: Intracoronary Abciximab and Aspiration Thrombectomy in Patients with Large Anterior Myocardial Infarction, AIDA-STEMI: Abciximab Intracoronary versus intravenous Drug Application in STEMI. MBG: Myocardial blush Grade
In a recent study,[54] combined use of GP 2B/3A inhibitors along with aspiration and balloon inflation, in STEMI cases resulted in reduced NR.
Intracoronary fibrinolysis
Though this treatment looks promising, further strong evidence is needed for their use in NR in PPCI.
In STEMI patients, low dose intracoronary alteplase during primary PCI did not reduce MVO in the T-TIME trial,[55] a randomized trial
According to a meta-analysis by Alyamani et al.,[56] intracoronary thrombolysis was safe and effective
In the DISSOLUTION trial.[57] manual aspiration followed by Urokinase in STEMI patients with large thrombus burden, showed greater prevalence of TIMI 3 flow, better ST-segment resolution, and favorable MACE at 6 months
Adjunct intracoronary streptokinase improved infarct size and LVEF in STEMI patients in another small randomized study.[58]
Intracoronary Tenecteplase dissolved thrombus and improved microvascular flow in patient with high thrombus burden in acute ST elevation MI in small series and registries.[59,60]
From these studies, the use of these agents could be beneficial in selected cases. The dose of the agent varied from 1/3rd to 1/6th of the systemic dose in these studies. Results of two on-going trials evaluating the efficacy of reduced doses of either alteplase (STRIVE trial) or Tenecteplase (RESTORE-MI) are awaited.
Thrombus aspiration
Initial results with manual thrombectomy were promising in the TAPAS study, subsequent trials were all negative results (TASTE and TOTAL trial). According to the latest guidelines,[43] routine aspiration thrombectomy in primary PCI is contraindicated. Manual aspiration thrombectomy is reasonable in primary PCI for high thrombus burden (class IIb, level of evidence C). No benefit of rheolytic thrombectomy (ANGIOJET/XSIZER) in primary PCI.
Pressure-controlled intermittent coronary sinus occlusion (PICSO)
PICSO is a device to transiently occlude flow in the coronary sinus, which increases the cardiac venous pressure and improve microcirculatory perfusion.[61] Use of PICSO before deploying the stent in patients with IMR more than 40 showed lesser infarct extension at 6 months in the Ox AMIPICSO trial.[62]
FUTURE INSIGHTS
Therapeutic hypothermia (TH)
It proved beneficial in reducing NR and myocardial damage in acute myocardial infarction in animal studies. However, the same benefit has not been shown in human trials. There are multiple reasons predicted to why the effective TH in animal study could not be seen in human trials-
Methods to cool the entire body even with the most effective cooling device take too long thus prolonging the ischemic time which counteracts its benefit. Target temperature could not be reached before reperfusion
Systemic hypothermia had many side effects – shivering in nearly all patients and even serious atrial arrhythmias in many
Hypothermia is effective when initiated before reperfusion, it is anticipated that in the studies conducted nearly 30% already have reperfusion and thus reperfusion injury before cooling is started.
TH appears to be a promising technique and further trials are necessary overcoming the above limitations. Results of the on-going EURO-ICE trial[63] (European Intracoronary Cooling Evaluation in Patients with ST-Elevation Myocardial Infarction), a prospective, randomized, and controlled trial evaluating the efficacy of hypothermia in humans is awaited. This is the largest study of hypothermia in humans where selective intracoronary hypothermia has been used during PPCI.
Selective Intracoronary Hypothermia
The occlusion site is first crossed with a regular guidewire and an OTW balloon inflated at the site of the occlusion.
A pressure/temperature wire (Pressure Wire™ X; Abbott, St. Paul, MN, United States of America) is placed into the distal coronary, and the guidewire is removed. Two infusion pumps are attached to the lumen of balloon-one filled with saline at room temperature (solution A) and the other with saline 4°C (solution B).
Solution A is infused for 7–10 min at a flow rate of 15– 30 mL/min (to maintain distal coronary at 6–8°C below body temperature) – occlusion phase
The OTW Balloon is deflated, and solution B infused for another 7–10 min-reperfusion phase. Flow rate adjusted to maintain the temperature in distal coronary 4–6°C below body temperature
The OTW balloon is removed, and stent deployed over the Pressure Wire X
Study in animals showed presence of temperature gradient during the occlusion phase, but not in the reperfusion phase.[64,65] The technique is being tested in humans in the EURO-ICE trial.
Hyperoxemic reperfusion (HR)
HR therapy is a treatment in which supersaturated oxygen is administered to a patient with STEMI following PCI to reduce myocardial damage. The procedure uses a device to remove arterial blood from a person, supersaturates it with oxygen and then reintroduce the highly oxygenated blood into the person’s affected coronary artery after stent deployment.
TherOx Downstream® System (TherOx Inc. of Irvine, CA) was approved in April 2019, for administration of super saturated oxygen therapy (SSO2 Therapy) following PCI to the left anterior descending coronary artery within 6 h of acute anterior wall MI
This technique when given for 90 min after PCI documented reduction in infarct size in the AMIHOT I[66] and AMIHOT II[67] studies; however, there was increased bleeding
In IC-HOT study,[68] the same administered for a duration of about 60 min proved safe with similar beneficial results.
Targeted anti-inflammatory approach and cellular approach for prevention and treatment of NR are presently in experimental stages. These include interventions that activate intracellular cardio protective signaling pathways directed at halting reperfusion injury. They have shown to reduce ischemic injury and improve myocardial perfusion following PCI and hold great promise. These techniques need refinement and further research is recommended before implementing them.
SIGNIFICANCE OF NR
NR phenomenon is associated with poor outcome immediately and in the long run. It underscores the benefits of PCI and is a nightmare for an interventional cardiologist. In-hospital course is complicated by life-threatening arrhythmias, heart failure and re-MI and hospitalization is prolonged. NR causes negative remodeling of the LV resulting in LV dilatation and heart failure leading to repeated hospitalizations and high 30-day mortality. Five-year survival is also low with high mortality (18.2% vs. 9.5%) in patients with NR compared to those with normal flow.
CONCLUSION
NR is an undesirable event that can complicate percutaneous coronary interventions especially in the setting of STEMI which could disheartens any interventional cardiologist. Prevention is better than cure hold true in this respect as, no available treatment is 100% effective. Different combinations of pharmacological vasodilation and antiplatelet therapy should be used in no specific order to achieve reversal of NR. Further research is mandatory to identify techniques to treat no-reflow that could benefit the outcome in these patients.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- The 'no-reflow' phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496-508.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine. 2020;99:e20152.
- [CrossRef] [PubMed] [Google Scholar]
- State of the art: No-reflow phenomenon. Cardiol Clin. 2020;38:563-73.
- [CrossRef] [PubMed] [Google Scholar]
- Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152-6.
- [CrossRef] [PubMed] [Google Scholar]
- Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10:215-23.
- [CrossRef] [PubMed] [Google Scholar]
- A risk score for no reflow in patients with ST-segment elevation myocardial infarction after percutaneous coronary intervention. Clin Cardiol. 2015;38:208-15.
- [CrossRef] [PubMed] [Google Scholar]
- Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: Predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122:1322-32.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet/lymphocyte ratio was associated with impaired myocardial perfusion and both in-hospital and long-term advers outcome in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Postepy Kardiol Interwencyjnej. 2015;11:288-97.
- [CrossRef] [PubMed] [Google Scholar]
- Role of moesin, Src, and ROS in advanced glycation end product-induced vascular endothelial dysfunction. Microcirculation. 2017;24:e12358.
- [CrossRef] [PubMed] [Google Scholar]
- Paraoxonase-1 activity and oxidative stress in patients with anterior ST elevation myocardial infarction undergoing primary percutaneous coronary intervention with and without no-reflow. Atherosclerosis. 2014;234:415-20.
- [CrossRef] [PubMed] [Google Scholar]
- Does serum bilirubin level on admission predict TIMI flow grade and in-hospital MACE in patients with STEMI undergoing primary PCI. Angiology. 2013;65:198-204.
- [CrossRef] [PubMed] [Google Scholar]
- The prognostic value of MELD-XI in elderly patients with ST-segment elevation myocardial infarction: An observational study. BMC Cardiovasc Disord. 2021;21:53.
- [CrossRef] [PubMed] [Google Scholar]
- MELD-XI score predict no-reflow phenomenon and short-term mortality in patient with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. BMC Cardiovasc Disord. 2022;22:113.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in coronary no-reflow phenomenon-a contemporary review. Curr Atheroscler Rep. 2018;20:44.
- [CrossRef] [PubMed] [Google Scholar]
- Mild to moderate renal impairment is associated with no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. Angiology. 2015;66:644-51.
- [CrossRef] [Google Scholar]
- The CHADS-VASc score is a predictor of no-reflow in patients with non-ST segment elevation myocardial infarction. Coron Artery Dis. 2020;31:7-12.
- [CrossRef] [PubMed] [Google Scholar]
- New R2-CHA2DS2-VASc score predicts no-reflow phenomenon and long-term prognosis in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Front Cardiovasc Med. 2022;9:899739.
- [CrossRef] [PubMed] [Google Scholar]
- Long non-coding RNA: Classifcation, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82-92.
- [CrossRef] [PubMed] [Google Scholar]
- The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biol. 2017;14:1705-14.
- [CrossRef] [PubMed] [Google Scholar]
- Propofol facilitates cisplatin sensitivity via lncRNA MALAT1/miR-30e/ATG5 axis through suppressing autophagy in gastric cancer. Life Sci. 2020;244:117280.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between circulating miRNA-30e and no-reflow phenomenon in STEMI patients undergoing primary coronary intervention. Scand J Clin Lab Invest. 2018;78:318-24.
- [CrossRef] [PubMed] [Google Scholar]
- Association of endothelial microparticle with NO, eNOS, ET-1, and fractional flow reserve in patients with coronary intermediate lesions. Biomarkers. 2015;20:429-35.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between plasma miR-126 and coronary slow flow phenomenon. Zhonghua Yi Xue Za Zhi. 2019;99:1323-7.
- [Google Scholar]
- LncRNA MALAT1 functions as a biomarker of noreflow phenomenon in STsegment elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Sci Rep. 2022;12:3294.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. 2018;48:e12928.
- [CrossRef] [PubMed] [Google Scholar]
- hsCRP and ET-1 expressions in patients with no-reflow phenomenon after percutaneous coronary intervention. Pak J Med Sci. 2017;33:920-5.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of no-reflow after percutaneous coronary intervention for culprit lesion with plaque rupture in infarct-related artery in patients with acute myocardial infarction. J Cardiol. 2009;54:36-44.
- [CrossRef] [PubMed] [Google Scholar]
- Resolution of ST-segment depression: A new prognostic marker in ST-segment elevation myocardial infarction. Eur Heart J. 2010;31:573-81.
- [CrossRef] [PubMed] [Google Scholar]
- Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc Imaging. 2009;2:1187-94.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of creatine kinase, CK-MB, myoglobin, and troponin T time-activity curves for early assessment of coronary artery reperfusion after intravenous thrombolysis. Circulation. 1993;87:1542-50.
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial contrast echocardiography: A 25-year retrospective. Circulation. 2008;118:291-308.
- [CrossRef] [PubMed] [Google Scholar]
- The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: Results of the multicenter AMICI study. J Am Coll Cardiol. 2008;51:552-9.
- [CrossRef] [PubMed] [Google Scholar]
- The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932-6.
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial contrast echocardiography with a new calibration method can estimate myocardial viability in patients with myocardial infarction. J Am Coll Cardiol. 2004;43:1799-806.
- [CrossRef] [PubMed] [Google Scholar]
- TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation. 1996;93:879-88.
- [CrossRef] [PubMed] [Google Scholar]
- Invasive assessment of the coronary microcirculation in reperfused ST-segment-elevation myocardial infarction patients: Where do we stand? Circ Cardiovasc Interv. 2017;10:e004373.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary microvascular resistance: Methods for its quantification in humans. Basic Res Cardiol. 2009;104:485-98.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436-41.
- [CrossRef] [PubMed] [Google Scholar]
- Index of microcirculatory resistance at the time of primary percutaneous coronary intervention predicts early cardiac complications: Insights from the OxAMI (Oxford study in acute myocardial infarction) Cohort J Am Heart Assoc. 2017;6:e005409.
- [CrossRef] [PubMed] [Google Scholar]
- Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129-32.
- [CrossRef] [PubMed] [Google Scholar]
- Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int J Cardiovasc Imaging. 2020;36:1395-406.
- [CrossRef] [PubMed] [Google Scholar]
- 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119-77.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462-9.
- [CrossRef] [PubMed] [Google Scholar]
- Contrast agents and cardiac MR imaging of myocardial ischemia: From bench to bedside. Eur Radiol. 2006;16:1951-63.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction: Recent advances, controversies, and future directions. Circulation. 2018;137:1949-64.
- [CrossRef] [PubMed] [Google Scholar]
- Integration of infarct size, tissue perfusion, and metabolism by hybrid cardiac positron emission tomography/ computed tomography: Evaluation in a porcine model of myocardial infarction. Circ Cardiovasc Imaging. 2009;2:299-305.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical implications of no reflow demonstrated with intravenous perfluorocarbon containing microbubbles following restoration of thrombolysis in myocardial infarction (TIMI) 3 flow in patients with acute myocardial infarction. Am J Cardiol. 1998;82:1173-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: The STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3:332-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: The SECURE-PCI randomized clinical trial. JAMA. 2018;319:1331-40.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the effects of ticagrelor and clopidogrel on microvascular dysfunction in patients with acute coronary syndrome using invasive physiologic indices. Circ Cardiovasc Interv. 2019;12:e008105.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of preoperative loading dose ticagrelor and clopidogrel on no-reflow phenomenon during intervention in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:2039-49.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet inhibition to target reperfusion injury trial: Rationale and study design. Clin Cardiol. 2019;42:5-12.
- [CrossRef] [PubMed] [Google Scholar]
- The “MAP strategy” (Maximum aspiration of atherothrombus and adjunctive glycoprotein IIb/ IIIa inhibitor utilization combined with prolonged inflation of balloon/stent) for preventing no-reflow in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: A retrospective analysis of seventy-one cases. Indian Heart J. 2015;67(Suppl 3):S43-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of low-dose intracoronary alteplase during primary percutaneous coronary intervention on microvascular obstruction in patients with acute myocardial infarction: A randomized clinical trial. JAMA. 2019;321:56-68.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of intracoronary thrombolysis as adjunctive therapy to primary PCI in STEMI: A systematic review and meta-analysis. Can J Cardiol. 2021;37:339-46.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of local delivery of thrombolytics before thrombectomy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (the delivery of thrombolytics before thrombectomy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention [DISSOLUTION] randomized trial. Am J Cardiol. 2013;112:630-5.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of intracoronary streptokinase administered immediately after primary percutaneous coronary intervention on long-term left ventricular infarct size, volumes, and function. J Am Coll Cardiol. 2009;54:1065-71.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of adjunctive intracoronary thrombolytic therapy during complex percutaneous coronary intervention: Initial experience with intracoronary tenecteplase. Cathet Cardiovasc Interv. 2005;66:327-32.
- [CrossRef] [PubMed] [Google Scholar]
- Intracoronary thrombolysis in patients with ST-segment elevation myocardial infarction presenting with massive intraluminal thrombus and failed aspiration. Eur Heart J Acute Cardiovasc Care. 2014;3:229-36.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Pressure-controlled intermittent coronary sinus occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. Int J Cardiol Heart Vasc. 2020;28:100526.
- [CrossRef] [PubMed] [Google Scholar]
- Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford acute myocardial infarction-pressure-controlled intermittent coronary sinus occlusion study (OxAMIPICSO study) EuroIntervention. 2018;14:e352-9.
- [CrossRef] [PubMed] [Google Scholar]
- Selective intracoronary hypothermia in patients with ST-elevation myocardial infarction. Rationale and design of the EURO-ICE trial. EuroIntervention. 2021;16:1444-6.
- [CrossRef] [PubMed] [Google Scholar]
- Intracoronary hypothermia for acute myocardial infarction in the isolated beating pig heart. Am J Transl Res. 2017;9:558-68.
- [Google Scholar]
- Safety and feasibility of selective intracoronary hypothermia in acute myocardial infarction. EuroIntervention. 2017;13:e1475-82.
- [CrossRef] [PubMed] [Google Scholar]
- Acute myocardial infarction with hyperoxemic therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:397-405.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ Cardiovasc Interv. 2009;2:366-75.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter Cardiovasc Interv. 2019;93:882-90.
- [CrossRef] [PubMed] [Google Scholar]







