Translate this page into:
The Gamut of Coronary Artery Disease in Indian Women
*Corresponding author: I. B. Vijayalakshmi, Department of Pediatric Cardiology, Super Specialty Hospital (Pradhana Mantri Swasthya Suraksha Yojana), Bengaluru Medical College and Research Institute, Bengaluru, Karnataka, India. docvj@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Vijayalakshmi IB, Nemani L, Kher M, Kumar A. The gamut of coronary artery disease in Indian women. Indian J Cardiovasc Dis Women 2023;8:43-51.
Abstract
Coronary artery disease is the leading cause of death among women. Majority of women suffering from CAD have one or more risk factors for CAD in their parents. Women are at higher risk for cardiac events with respect to traditional risk factors including dyslipidemia, hypertension, diabetes, and smoking. Menopause, pregnancy complications, inflammation, anemia, migraines, and depression are important sex-specific novel risk factors for CVD, and it is important that clinicians should be aware of these risks to design strategies for prevention. Education, self-awareness in women, and timely recognition of CAD in women with lifestyle modifications and timely intervention result in better outcomes.
Keywords
Coronary artery disease
Women
Risk factors
INTRODUCTION
In the present scenario, coronary artery disease (CAD) is the leading cause of death among women. It is the cause of death for one in three women every year, killing approximately one woman every minute. Majority of women (90%) suffering from CAD have one or more risk factors for CAD in their parents.[1] Coronary heart disease (CHD) continues to be prime killer and crippler in India – this was commented way back in 1995 in a chapter titled[2] “Coronary Artery Disease in Indian Women Change the Gender Bias” by me. The incidence of CAD in India increased from 1.05% in 1960 to 9.67% in 1995. The greatest rise was seen in men below 40 years and women below 50 years.[3] Mortality from CAD was also high in Indian men and women (36% and 46% higher, respectively) compared to general population in England. The incidence of CAD according to the WHO will be doubled by the year 2020 thus making CAD as the leading cause of death among men and women.
Sadly, the prediction of 1995 has already come true in 2021. The article titled “Gender differences in South Indians with premature CAD (<40 years) – Insights from the PCAD registry” states – “Nearly one-third of all fatalities are caused by CAD, which is also the major cause of morbidity and mortality in both sexes.”[4] It accounts for higher death rate in women, regardless of their race or ethnicity.[5] CAD being an epidemic today among Indians is nearly doubling in the incidence compared to the Americans and several fold higher than other Asians.[6] Indian ethnicity is now considered a risk factor by itself.[7] According to statistics, there has been a rise in proportionate cardiovascular disease (CVD) mortality, with 20.6%, 21.4%, 24.3%, 27.5%, 29.0% of deaths occurring in 1990, 1995, 2000, 2005, and 2013, respectively.[8] According to the Registrar General of India, CAD accounted for 26.9% of all medically certified fatalities in 2015 and, circulatory system disorders representing one-third of these deaths.[9] In India, the average age of the first myocardial infarction (MI) has dropped by 20 years. Half of all MI occur in people under the age of 50 and 25% under the 40 years of age. It has been determined that age-standardized values for the number of disability-adjusted life years lost as a result of CAD per 1000 people in India are triple times higher than in industrialized countries.
Indians have “Malignant CAD” – severe and extensive coronary atherosclerosis affecting young individuals (≤45 years in men and ≤50 years in women) with very low burden of established risk factors. It is important to analyze gender differences in risk factors, clinical presentation, and angiographic profile in Indian youth from the on-going premature CAD registry which is very important. Hence, awareness of the whole gamut of CHD is of paramount importance to prevent CHD in Indian women.
FRAMINGHAM HEART STUDY: TRUTH AND MYTH
The concepts and fundamentals that women are less affected by CAD came from landmark Framingham Heart Study (FHS).[10] The study began in 1948 with 5209 adult subjects from Framingham and is now on its third generation of participants. The pathophysiology of cardiac disease occurs from the results of FHS studies. The risk of traditional risk factors such as hypertension, diabetes mellitus, and tobacco consumption is similar for both sexes. Menopause was found to increase the risk of CAD in women and psychosocial factors found to affect women. This misleads physicians that younger women are immune to CAD due to hormonal protection and chest pain in women is largely due to psychological stress. This is the root cause for major gender bias in CAD. Prognosis of Framingham women with angina was more favorable than that of men. While 75% of the men with angina developed acute MI within 5 years, only half of women with angina developed infarction in Framingham study, which made things even worse and created gender bias.
EPIDEMIOLOGY AND PREVALENCE OF CAD AMONG INDIAN WOMEN
Epidemiologic and clinical research shows important gender differences in the prevalence, presentation, risk factors, management, and outcomes of CAD patients. Indian adults’ prevalence of CAD grew from 1960 to 1995 from 2% to 4% and 3% to 10% in rural and urban Indians, respectively, with rates comparable to men.[11] In a rural community in central India, the prevalence of CAD in those over 60 years old was 51.3/1000, with nearly equal sex distribution, and hypertension was shown to be the only risk factor.[11] CAD incidence was higher in women and more deaths are seen due to heart disease than breast cancer. Heart disease in women accounts to more than 40% of all deaths. Since 1984, more women than men have died every year from CAD.[12] Higher mortality and worse prognosis are seen in women after acute cardiovascular events be it CHD, stroke, heart failure, or aortic diseases. Sex-specific differences in pathophysiology, prevalence, and impact of CVD risk factors may explain the high cardiovascular mortality rates in women. As CAD is a leading cause of death in post-menopausal women than all cancers combined,[13] very few studies in India have addressed the problems of CAD among Indian women.
GENDER DIFFERENCES AND BIAS
At first presentation, women with CAD are older than men and have more cardiovascular risk factors.[14,15] CAD remained the leading cause of death for women over the age of 65 despite developing 7–10 years later in women than in men. According to statistics from the National Health and Nutrition Examination Survey, midlife women (aged 35–54) have a higher prevalence of MI than older males over the past two decades.[16] According to the European Heart Survey report on stable angina pectoris, much less women are referred for functional testing for ischemia and less likely to undergo diagnostic angiograms and interventional procedures as compared with men[2] due to the different clinical presentation in women. Under-recognition of heart disease also leads to less aggressive therapeutic approaches and a smaller proportion of female participants in clinical trials.
DIFFERENCES IN PATHOPHYSIOLOGY
MI with non-obstructive coronary arteries reported between 5.9% and 11% is much more common presentation in women, especially younger and non-white patients.[17,18] Angiographically normal coronaries in a young women presenting with acute coronary artery syndrome (ACS) are attributed to coronary microvascular dysfunction by aberrant inflammatory responses, low endogenous estrogen levels, coagulation disorders, and endothelial reactivity.[19] Increased pain perception in women could probably explain the abnormal atypical persistent cardiac pain in them.[20] Epicardial atherosclerosis and microvascular dysfunction relationship is still unclear.
Estrogens have a regulating effect on coagulant system, lipids, and the inflammatory markers. They have a direct vasodilatory effect on the vessel wall.[21] Women can exhibit subclinical atherosclerosis signs as shown by intima-media thickness measurements even before menopause, particularly in the presence of coronary risk factors.[22] Flow-mediated vaso-reactivity decreases with progression of time following menopause. Atherosclerotic plaque composition evolves into more vulnerable lesions with menopause.
Do women have a specific Genetic code? Women’s MI has been linked to stromelysin-1, a member of the matrix metalloproteinase family of enzymes implicated in plaque rupture and plasminogen activator inhibitor-1.[23] Recent research has identified due to MEF2A’s high expression in the endothelium and its role in the development of CAD and MI, it hypothesizes endothelial dysfunction as an early trigger for MI and CAD. The available genome-wide association studies do not show any sex bias.
RISK FACTORS
The so called traditional risk factors (tobacco use, unhealthy diet, obesity, physical inactivity, hypertension, diabetes, hyperlipidemia, and harmful use of alcohol) have been identified as cause of CAD and stroke in both genders.
Age
It was said that women have been at a 10 year advantage over men in risk for CAD. Unfortunately, smoking, diabetes, and premature menopause have nullified this difference.
Family history
Sisters who have a history of CAD are at a 12-fold higher risk than brothers and parents, who are each at a risk of six-fold and three-fold, respectively. A very substantial risk factor is the sister’s history of MI or sudden cardiac death when she was aged <55.
Smoking
Women have an increased risk of AMI due to smoking than men. Smoking down regulates estrogen-dependent vasodilatation of the endothelium.[24] In the young (<50 years), smoke consumption is four-fold dangerous in women than men.[25] In the 21st century, tobacco consumption (smoking beedies, snuff, and chewing tobacco) is a significant risk factor for CAD in Indian (CADI) women; however, the risk is on an increase. Only 8% women compared to 60% men smoke in Asia.[26] Passive smoking is a major issue in India which increases the risk of fatal and non-fatal cardiac events. It is known that the risk of CAD begins to decline within months of cessation of smoking and almost disappears within 3–5 years. The need of the hour is most important health measures to improve the risk posed by both active and passive smoking in India.
Socioeconomic status
The gradual rise in CVD risk factors in rural and slum populations across India represents a significant shift in risk factor dynamics.[27,28] There is increase in smoking and non-smoked tobacco,[27] sedentariness has crawled in,[29] and there is change in dietary habits. High-calorie fast foods (comfort foods) are easily available, there is greater consumption of harmful fats and processed foods.[30] Mortality due to CAD is also higher (30–50% higher) in women with low education standards.
DIABETES MELLITUS
According to the National Cholesterol Education Program Adult Treatment Panel III, diabetes is an analogous CAD risk factor.[31] Compared to men, it is a substantially bigger risk factor for CAD in women. Women with diabetes experience CAD twice as frequently as those without. It is associated with three- to seven-fold higher incidence of CAD and two- to three-fold higher risk of death rate in men. Women who have diabetes are more likely to have heart failure (eight-fold vs. four-fold) compared to men. Diabetes eliminates the protective effects of estrogens and predisposes to premature CAD.[32]
DYSLIPIDEMIA
Compared to premenopausal women, postmenopausal women have worse lipid profiles. When compared to premenopausal women of the same age, menopausal women had levels of total cholesterol (TC) and low-density lipoprotein (LDL) that were 0.2–0.4 and 0.1–0.3 mmoL/l higher, respectively, according to a cross-sectional research of 63,466 women, aged 18–65. Postmenopausal women had smaller LDL particle size. There was no significant change in VLDL or high-density lipoprotein (HDL) concentrations. Thus, both chronological aging and menopause predispose to dyslipidemia. A lifetime risk of developing CAD is increased by 50–60% with a 20% increase in TC level.[33]
Low HDL is a potent predictor of CADI women after onset of 65 years. Indian women have very low levels of HDL than other ethnic groups which parallel their high rates of CAD.[34] In the CADI study,[35] 70% of Indian women had HDL level <50 mg/dL. HDL-c level <35 mg/dL confers an eight-fold higher CAD risk.[36]
TC/HDL ratio more than 5 is the single best predictor of CAD in both men and women and is seen in 25% of industrial and 32% of urban Indian women[37]
Compared to men, women have a higher propensity for CAD when their TG levels are high. In addition, a TG level increase of 90 mg/dL increases the risk of CAD in women by 75% as opposed to 30% in males.[38]
Due to their low HDL, and high TG, and small dense LDL levels, Indian men and women are hence more vulnerable to CAD.[39]
NOVEL RISK FACTORS
Lipoprotein (a)
Elevated Lp (a) is a powerful risk factor for premature CAD and mortality in both men and women, but more in premenopausal women. In association with hypertension it shows five-fold increased cardiac risk, seven-fold with concomitant high TC/HDL ratio, eight-fold with low HDL, and nine-fold with high homocysteine. A combination of all the above increases the risk of CAD by a factor of 122.[40] Lipoprotein (a) levels show increase by 10% in postmenopausal and decrease by 20% with hormone replacement therapy.
Migraines
Aura-accompanied migraines have previously been linked to a higher risk of ischemic stroke. The risk of CAD is higher in migraine-prone women, according to recent studies.[41] The potential mechanisms include a higher risk of thrombotic events, inflammation, and association with adverse cardiovascular risk factors such as obesity, hyperlipidemia, and hypertension.
Anemia
It is well known that anemia especially iron deficiency anemia is common in Indian women and has been associated with poor outcomes in both acute coronary syndrome and heart failure.
Depression and other psychosocial factors
Majority of women have psychological stress, due to rapid urbanization and nuclear families, the incidence has increased in both working women and housewives. Stress-induced release of catecholamine causes acceleration of heart rate, blood pressure, vasoconstriction, leading to demand-supply mismatch which contributes to ischemia. Depression and panic disorders[42,43] in women with CAD have adverse effects in terms of morbidity and mortality. Timely, recognition and treatment of mental stress can avoid CAD and its poor outcomes. Although chest pain and tachycardia can be features of depression and anxiety in women, physicians should access these symptoms and not diagnose this as a psychogenic origin without thorough investigations [Figure 1].

- Pathophysiologic mechanisms of MINOCA and INOCA.
AUTOIMMUNE DISEASES
Autoimmune diseases such as rheumatoid arthritis and SLE are more common in women predisposing to increased cardiovascular morbidity and mortality due to accelerated atherosclerosis seen in young women.
RISK FACTORS UNIQUE TO WOMEN
Some risk factors are specific to women – (1). Primary ovarian insufficiency, (2). pregnancy-induced hypertension, (3). pre-eclampsia, (4). gestational diabetes mellitus, (5). preterm birth, and (6). polycystic ovary syndrome and menopause.[44]
Pregnancy
Pregnancy in women is an analyzer of their CAD future risk. The various studies shown a higher risk of CAD with multiparty even after controlling for established CVD risk factors, and a meta-analysis has shown “J-shaped” relationship among number of pregnancies and later CAD. Pregnancy-induced hypertension, preeclampsia, gestational diabetes mellitus, and adverse outcome during pregnancy including preterm birth <37 weeks have proved to be strong risk factors for CAD.
Multiple pregnancy complications and their severity seem to be related to a higher chance of developing CAD. Women who also had preeclampsia with pre-existing hypertension had a nearly six-fold higher risk compared to four-fold risk of pre-existing hypertension alone, according to a large pregnant cohort study.[45]
Menopause and premature menopause
In women, exposed to endogenous estrogen during the reproductive years prevents the development of atherosclerosis. Several metabolic variables, including coagulant system, inflammatory indicators, and lipids, are controlled by estrogens. The risk profile for CHD deteriorates with menopause,[46] and the incidence of CAD increases substantially. Women who experience an early menopause (before the age of 40) have a 2-year shorter life expectancy than those who experience a typical menopause. According to the FHS, the dangerous cardiovascular risk profile is more than menopause age for CAD. According to the women’s ischemia syndrome evaluation (WISE) study, endogenous estrogen increases cardiovascular risk in young women 7 times.[47]
CAD IN PREGNANCY
Ischemic heart disease in pregnancy is rare, 2.8 and 6.2/100,000 deliveries. Incidence is on a rise due to advanced maternal age with its associated medical comorbidities. Pregnancy itself seems to be a risk factor for AMI, although the exact mechanisms are not clear. AMI in pregnancy should be investigated in the same manner as in the non-pregnant population. Maternal morbidity following AMI is high due to increased rates of heart failure, arrhythmia, and cardiogenic shock during pregnancy.[4]
SPONTANEOUS CORONARY ARTERY DISSECTION (SCAD)
SCAD is a rare cause of ACS (0.1–4%) and sudden cardiac death (0.4%) and is seen in 0.2–1.1% of coronary angiograms in the general population.[46] SCAD is the most common non-atherosclerotic cause of CAD in young women, accounting for 24% of MI, according to Douglas PS et al.,[48] who analyzed coronary angiograms of women under 50 from 2009 to 2011.[48]
CLINICAL PRESENTATION AND NON-INVASIVE TESTING
Women are less reliable than men when it comes to the clinical manifestation of CAD and non-invasive diagnostic testing, particularly in the age range under 55 when the prevalence of CAD is still low.[48-50]
Women have chest pain syndromes more frequently than males do, and they are less often linked to atherosclerosis in the large epicardial coronary arteries.[51] These women have a 4 times higher chance of re-hospitalization and repeat angiograms during the next 180 days, as well as a two-fold increased risk of cardiac event over the course of 5–7 years. A defined clinical event like MI is less common in women. Apart from chest pain, women have symptoms such as nausea, sweating, vomiting, pain in the neck, jaw, throat, abdomen, or back more than men. The classification of typical angina would miss 65% of women with CAD (according to WISE) study and is labeled as non-ischemic chest pain. Furthermore, several causes of non-cardiac chest pain can mimic the discomfort that is related with MI. The implication is that traditional diagnostic methods are not optimal for women and assessment and diagnosis should be based on the importance of risk factor profile at presentation [ Chart 1].
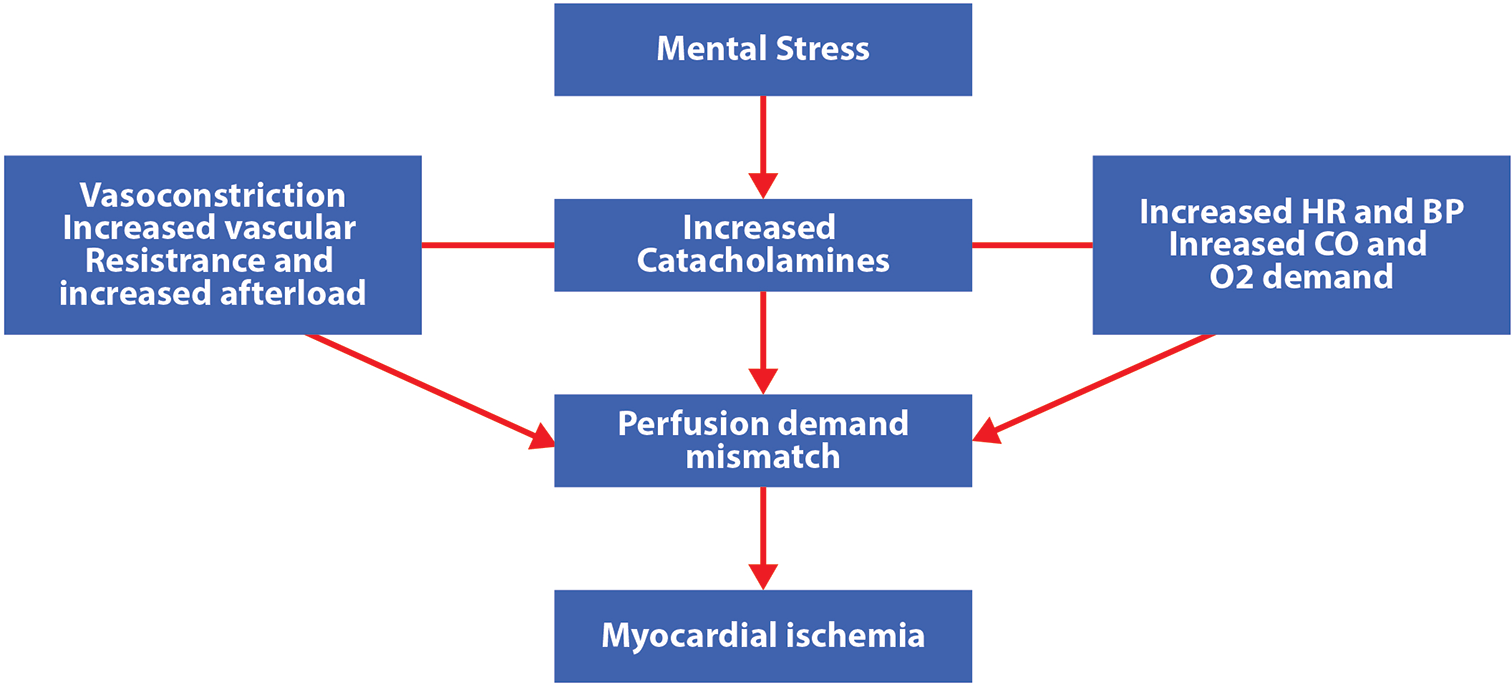
- The mechanism of ischemia occurring due to stress.
ELECTROCARDIOGRAPHY
While women exhibit tachycardia at rest and a longer QT interval, there are no gender-specific criteria for ECG interpretation.
An ECG showing a 15–20 leftward axis deviation during pregnancy indicates that the heart rotates to the left. Shifts that are frequently observed include transient ST/T wave changes, attenuated Q waves in lead aVF, Q waves and inverted T waves in lead III, and inverted T waves in V1, V2, and infrequently V3. These could resemble structural cardiac problems such as LV hypertrophy.
EXERCISE TESTING
Non-specific ECG changes at rest, lower exercise capacity, and smaller vessel size contribute to the lower sensitivity and specificity of non-invasive testing in women.[49] It has been observed that endogenous estrogen might cause ECG abnormalities in young women that mimic ischemia. For treadmill exercise assessment, normograms specifically for women have been developed. A limited capacity for exercise in both symptomatic and asymptomatic women is a reliable indicator of 5-year mortality. By evaluating the angina history, estrogen status, and presence of the main CHD risk factors, accuracy in the diagnostic capability of exercise testing in women can be further improved.[52]
HOLTER MONITORING
Holter monitoring is a reliable indicator of arrhythmias and should be used in individuals who have a history of palpitations or VT, AF, or paroxysmal or persistent arrhythmias.
CORONARY ANGIOGRAM
Men typically have a plaque in major epicardial vessels, but the plaque in women more commonly is in the smallest blood vessels, known as the microvasculature. ACS with angiographically “normal” coronaries is more common in young women than men (41 vs. 8% in CASS study). This coronary microvascular dysfunction is assumed to be related to endothelial reactivity, low endogenous estrogen levels, coagulation disorders, and abnormal inflammation.[53] In addition, abnormal cardiac nociception attributes to persistent chest pain in women. Thus, the symptoms of microvascular dysfunction result in objective signs of ischemia, which is addressed as micro vascular angina pectoris. It is yet unclear how epicardial atherosclerosis and microvascular dysfunction are related. This syndrome has a less favorable prognosis and frequently requires hospital stays and coronary angiograms.[54,55] The prognosis is worse in women and these should be treated aggressively to prevent future CHD events as a priority.
ACUTE CORONARY SYNDROMES IN WOMEN
The symptoms of chest pain in ST-elevation MI (STEMI) are similar in men and women, but women tend to have more concurrent vaso-vegetative symptoms, which might obscure the symptoms of chest pain, and less significant ST-T elevations at admission, particularly in younger patients.[56,57] Unstable angina pectoris and non-STEMI are frequently misdiagnosed in women under the age of 55 at the emergency department.[57] Women with ACS tend to be older and have more risk factors clustered together, which may increase their mortality risk.[58] It is commonly recognized that women have more diffuse and less extensive obstructive CAD than men, and that their event rates are also greater. In both genders, altogether management is the same.
A major area of concern is STEMI during pregnancy. Management relies on PCI. Thrombolysis can be a last resort, as recombinant tissue plasminogen activator is advised in non-pregnant individuals, because it does not cross the placenta but may result in bleeding complications (sub-placental bleeding). Catheterization procedures during pregnancy must follow the “as low as reasonably achievable” principle to minimize radiation exposure. Maneuvers that should be implemented are: (i) echo guidance whenever feasible; (ii) Anteroposterior projections; (iii) minimize fluoroscopy time; (iv) avoid direct radiation of the abdominal region; (v) source of radiation as far as possible from patient; (vi) collimate as tightly as possible to the area of interest; (vii) low-dose fluoroscopy; and (viii) utilize an experienced cardiologist.[53] The radiation exposure to the fetus can be somewhat reduced by abdominal shielding, but the presence of lead in the primary beam’s field may have the opposite effect by increasing scattered radiation. However, shielding should not interfere with an optimal intervention. Assessing potential impacts on the fetus in the future is made easier by tracking and recording radiation exposure.[54]
MORTALITY RATES IN WOMEN
Data from the FHS showed that CVD mortality has declined among women with diabetes; however, overall mortality has not declined. Thus diabetes is a greater risk factor for CVD mortality in women.[59-61] Mortality after CABG is higher in women especially in the young, after adjustment for risk factors.[62] Associated comorbidities, smaller vessels, need of urgent procedures in women, and the presence of hypertensive heart disease contribute to this. Bleeding complications are higher after PCI in women, especially when glycoprotein IIb/IIIa inhibitors are used.[63] In a meta-analysis of 11 randomized ACS trials, no differences were found in the efficacy and safety of clopidogrel between men and women.[64] Sex-based differences in 30-day mortality in ACS are based on atypical clinical presentation and the severity of angiographically documented disease.
LIFESTYLE MODIFICATION
The present hour requires preventive strategies to combat this epidemic. Education, self-awareness in women, and timely recognition of CAD in women with lifestyle modifications and timely intervention result in better outcomes.
CONCLUSION
CAD in India is like an epidemic which is escalating. Women are at higher risk for cardiac events with respect to traditional risk factors including dyslipidemia, hypertension, diabetes, and smoking. Menopause, pregnancy complications, inflammation, anemia, migraines, and depression are important sex-specific novel risk factors for CVD, and it is important that clinicians should be aware of these risks to design strategies for prevention. Pregnancy offers a unique opportunity and window to screen otherwise healthy women who are at an increased risk of CVD in the future. Indian women have a higher prevalence of hypertension, diabetes, obesity, and family history of CHD, yet lag timely intervention. There is dire need to promote primordial, primary, and secondary prevention strategies. Primordial strategies such as promotion of healthy dietary habits, physical activity, and smoking/tobacco cessation should prevent risk factors from occurring in the first place. To reduce the risk of overt CHD, primary prevention focuses on screening and better risk factor management. Morbidity can be avoided through effective secondary prevention and better acute and chronic event management. Excellent quality secondary and tertiary care will reduce morbidity and mortality as women are the strongest roots of a healthy society. Multicentric trials dedicated to women are the need of hour to prevent the disease.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Risk factors for coronary artery disease In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- [Google Scholar]
- Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021;143:410-23.
- [CrossRef] [PubMed] [Google Scholar]
- Takotsubo cardiomyopathy In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- [Google Scholar]
- Referrals in acute coronary events for cardiac catheterization: The RACE CAR trial. Can J Cardiol. 2010;8:e290-6.
- [CrossRef] [PubMed] [Google Scholar]
- Heart disease and stroke statistics-2006 update: A report from the American Heart association statistics committee and stroke statistics subcommittee. Circulation. 2006;113:e85-151.
- [CrossRef] [PubMed] [Google Scholar]
- How far can risk factors account for excess coronary mortality in South Asians? Can J Cardiol. 1997;13:47.B.
- [Google Scholar]
- Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The study of health assessment and risk in ethnic groups (SHARE) Lancet. 2000;356:279-84.
- [CrossRef] [PubMed] [Google Scholar]
- Report on Medical Certification of Cause of Death, 2007 In: Ministry of Home Affairs. New Delhi: Office of Registrar General; 2013.
- [Google Scholar]
- Office of the Registrar General. New Delhi, India. 2015. Available from: https://www.censusindia.gov.in/2011-document/mccd_2013.pdf [Last accessed on 2016 Jan 27]
- [Google Scholar]
- Legacy of the Framingham heart study: Rationale, design, initial findings, and implications. Glob Heart. 2013;8:3-9.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of confirmed coronary heart disease among population older than 60 years in rural central India-A community-based cross-sectional study. Indian Heart J. 2019;71:39-44.
- [CrossRef] [PubMed] [Google Scholar]
- Office of the Registrar General of India, Government of India Ministry of Home Affairs, Vital Statistics Division R. K. Puram New Delhi. 2015. Available from: https://www.censusindia.gov.in/2011-Documents/mccd_Report1/MCCD_Report-2015.pdf [Last accessed on 2018 Mar 01]
- [Google Scholar]
- The relationship between coronary calcification and the natural history of coronary artery disease. JACC Cardiovasc Imaging. 2021;14:233-42.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med. 2009;169:1762-6.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical characteristics and investigations planned in patients with stable angina presenting to cardiologists in Europe: From the euro heart survey of stable Angina. Eur Heart J. 2005;26:996-1010.
- [CrossRef] [PubMed] [Google Scholar]
- Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341:226-32.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines) Circ Cardiovasc Qual Outcomes. 2017;10:e003443.
- [CrossRef] [PubMed] [Google Scholar]
- Presentation, clinical profile, and prognosis of young patients with myocardial infarction with non obstructive coronary arteries (MINOCA): Results from the VIRGO study. J Am Heart Assoc. 2018;7:e009174.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary intervention in 2009. Are women no different than men? Circ Cardiovasc Interv. 2009;2:69-78.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent chest pain and no obstructive coronary artery disease. JAMA. 2009;301:1468-74.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic research and women's heart disease: A primer. Curr Atheroscler Rep. 2016;18:67.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29:1116-21.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916-23.
- [CrossRef] [PubMed] [Google Scholar]
- Refinement of variant selection for the LDL cholesterol genetic risk score in the diagnosis of the polygenic form of clinical familial hypercholesterolemia and replication in samples from 6 countries. Clin Chem. 2015;61:231-8.
- [CrossRef] [PubMed] [Google Scholar]
- Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: A case-control study. Lancet. 2013;381:1293-301.
- [CrossRef] [PubMed] [Google Scholar]
- Morbidity and mortality in relation to cigarette smoking in Shanghai, China: A prospective male cohort study. JAMA. 1996;275:1646-50.
- [CrossRef] [PubMed] [Google Scholar]
- Serial epidemiological surveys in an urban Indian population demonstrate increasing coronary risk factor among the lower socioeconomic strata. J Assoc Physicians India. 2003;55:470-7.
- [Google Scholar]
- Convergence in urban-rural prevalence of hypertension in India. J Hum Hypertens. 2016;30:79-82.
- [CrossRef] [PubMed] [Google Scholar]
- Conditions of SC/ST households: Story of unequal improvement. Econ Pol Wkly. 2013;48:62-6.
- [Google Scholar]
- Nutrition transition in India: Secular trends in dietary intake and their relationship to diet-related noncommunicable diseases. J Diabetes. 2011;3:278-92.
- [CrossRef] [PubMed] [Google Scholar]
- Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486-97.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes mellitus and cardiovascular disease in women. Arch Intern Med. 1998;158:617-21.
- [CrossRef] [PubMed] [Google Scholar]
- From journal to bedside: Quantifying the benefits of treatment. Evid Based Cardiovasc Med. 1997;5:7-58.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary artery epidemic in Indians: A cause for alarm and call for action. J Indian Med Assoc. 2000;98:694-702.
- [Google Scholar]
- Prevalence of coronary artery disease in Asian Indians. Am J Cardiol. 1992;70:945-9.
- [CrossRef] [PubMed] [Google Scholar]
- High density lipoprotein cholesterol, total cholesterol screening and myocardial infarction. The Framingham study. Arteriosclerosis. 1988;8:207-11.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596-601.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein levels: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213-9.
- [CrossRef] [Google Scholar]
- Plasma levels of apolipoproteins A-1 and B in Indian patients with angiographically defined coronary artery disease. Int J Cardiol. 1994;46:143-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lipoprotein(a) interactions with lipid and nonlipid risk factors in early familial coronary artery disease. Arterioscler Thromb Vasc Biol. 1997;17:2783-92.
- [CrossRef] [PubMed] [Google Scholar]
- Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i261.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological predictors of heart disease: A quantitative review. Psycho Bull. 1987;101:343-62.
- [CrossRef] [Google Scholar]
- Psychological factors and risk of ischemic heart disease and death in women in Gothenburg Sweden. J Psychasom Res. 1986;30:451-59.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in cardiovascular disease. Med Novel Technol Devices. 2019;4:100025.
- [CrossRef] [Google Scholar]
- Pregnancy complications and cardiovascular disease death: 50-year follow-up of the child health and development studies pregnancy cohort. Circulation. 2015;132:1234-42.
- [CrossRef] [PubMed] [Google Scholar]
- Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641-6.
- [CrossRef] [PubMed] [Google Scholar]
- The evaluation of chest pain in women. N Engl J Med. 1996;334:1311-5.
- [CrossRef] [PubMed] [Google Scholar]
- Role of non-invasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the cardiac imaging committee, council on clinical cardiology, and the cardiovascular imaging and intervention committee, council on cardiovascular radiology and intervention, American Heart Association. Circulation. 2005;111:682-96.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical risk assessment in women: Chest discomfort. Report from the WISE study In: Shaw LJ, Redberg RF, eds. CAD in Women: Evidence-based Diagnosis and Treatment. Totowa, NJ: Humana Press; 2003. p. :129-42.
- [Google Scholar]
- Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47:S30-5.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the accuracy of pretest and exercise test scores in women with a low prevalence of coronary disease: The NHLB sponsored Women's ischemia syndrome evaluation (WISE) study. Am Heart J. 2004;147:1085-92.
- [CrossRef] [PubMed] [Google Scholar]
- Women and ischemic heart disease. J Am Coll Cardiol. 2009;54:1561-7.
- [CrossRef] [PubMed] [Google Scholar]
- Angina with “normal” coronary arteries: Sex differences in outcome. Am Heart J. 2008;155:375-81.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease. A report from the women's ischemia syndrome evaluation study and the St James women take heart project. Arch Intern Med. 2009;169:843-50.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The global registry of acute coronary events. Heart. 2009;95:20-6.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction. Circulation. 2007;115:833-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes as a risk factor for acute coronary syndrome in women compared with men: A meta-analysis, including 10 856 279 individuals and 106 703 acute coronary syndrome events. Diabetes Metab Res Rev. 2017;33:e2887.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in the excess risk of cardiovascular diseases associated with Type 2 diabetes: Potential explanations and clinical implications. Curr Cardiovasc Risk Rep. 2015;9:36.
- [CrossRef] [PubMed] [Google Scholar]
- Gender difference in diabetes related excess risk of cardiovascular events: when does the “risk window” open? J Diabetes Complications. 2017;31:74-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in hospital mortality after coronary artery bypass surgery: Evidence for a higher mortality in younger women. Circulation. 2002;105:1176-81.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: Results from the CRUSADE initiative. Circulation. 2006;114:1380-7.
- [CrossRef] [PubMed] [Google Scholar]
- The relative efficacy and safety of clopidogrel in women and men. J Am Coll Cardiol. 2009;54:1935-45.
- [CrossRef] [PubMed] [Google Scholar]







