Translate this page into:
Marfan Syndrome with CRHD
Dr. Shahood Ajaz Kakroo, DM Department of Cardiology, Nizam’s Institute of Medical Sciences (NIMS) Punjagutta, Hyderabad-TS -500 082 India shahood.cardio@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Marfan syndrome (MFS) is an inheritable disorder caused by mutation of fibrillin-1 gene. It is the most common disorder among disorders of connective tissue. Its mode of inheritance is autosomal dominant. The reported prevalence of this disorder is one in three thousand (3000) to five (5000) thousand individuals. It presents with varied manifestation and different range of severity. The organ systems most commonly affected by this disorder include eyes, cardiovascular system, and musculoskeletal system.
The other systems which may be affected include respiratory system, skin, and central nervous system. It is diagnosed with the help of revised Ghent score which includes a set of various diagnostic criteria which need to be fulfilled. MFS in this patient was diagnosed after the fulfillment of the revised Ghent score criteria, which included a positive history of MFS in the family and a systemic score of 8.
In this case report, we are reporting a case of MFS which is unusual and remarkable in the sense that it is associated with chronic rheumatic heart disease (CRHD), and not the cardiovascular features which are usually present in cases of MFS. We tried to find a similar case if ever reported previously and, after extensive search, we could find only few cases13 14 15 of MFS which were associated with CRHD.
Keywords
MFS Marfan syndrome
CRHD Chronic rheumatic heart disease
Introduction
Marfan syndrome (MFS) is characterized by a range of clinical manifestations. Cardiovascular disorders form a common manifestation of this disorder. The various abnormalities usually associated include disorder of the aorta (thoracic and abdominal), which varies in severity from mild abnormal stiffness1 2 3 4 5 to more severe aortic aneurysm and sometimes dissection.
The mitral valve involvement includes elongation of the valve and thickening, usually myxomatous, leading to prolapse of the mitral valve and subsequent regurgitation. This involvement of the mitral valve is the commonest abnormality affecting valves in cases of MFS. The prolapse of mitral valve has a prevalence of 28 to 75 percent in cases of MFS with regard to 2.4 percent prevalence in the usual general population.6
Among the complications associated with MFS, particularly in children, it may be associated with mitral regurgitation of severe intensity which may, in turn, lead to dysfunction of the myocardium and subsequent heart failure.7 The mitral valve can be surgically corrected in patients who have severe regurgitation, but such patients are at an enhanced risk of thoracic aortic dissection, which may develop as a result of sudden rise of the cardiac output in the immediate postoperative period.8
Manifestations of MFS in the neonates includes insufficiency of the mitral/aortic valves, defects of the atrial septum (ASD), ventricular septum (VSD), and subsequent heart failure.9 10 The dysfunction of the left ventricle, systolic as well as diastolic, in patients afflicted with MFS in adulthood is usually due to insufficiency of the valve and overload (volume) of the left ventricle.
The increased stiffness of the arterial walls alters the hemodynamic parameters which subsequently leads to cardiac dysfunction. Several research works11 have demonstrated presence of abnormal functioning myocardium when other causes like mitral valve prolapse or thoracic aortic aneurysm leading to myocardial dysfunction are not present.
Early detection of the myocardial dysfunction, particularly in patients who do not have symptoms includes use of novel echo methods or techniques. The gene mutation severity present in MFS is directly related to the left ventricular (LV) dysfunction which suggests a possibility of cardiomyopathy in these patients. Further studies are required to predict the value with regard to prognosis of these observational studies.12
Case Report
A 55-year-old male, normotensive, nondiabetic, alcoholic, and also afflicted with chronic obstructive pulmonary disease COPD, presented with history of shortness of breath for the last 2 months. Initially, the patient was able to do his routine activities, but then he felt breathless even on rest with history of paroxysmal nocturnal dyspnea PND attacks during the night over the last 20 days. There was also history of cough with expectoration and blood streaking. There was no history of orthopnea, palpitations, pedal edema, syncope, and fever. There was history of fever with joints pains during childhood which subsided on its own and was not evaluated. The patient had no other significant history of illness in the past. A positive history of MFS was present in the family. There is no significant history of any regular medications or injections in the past.
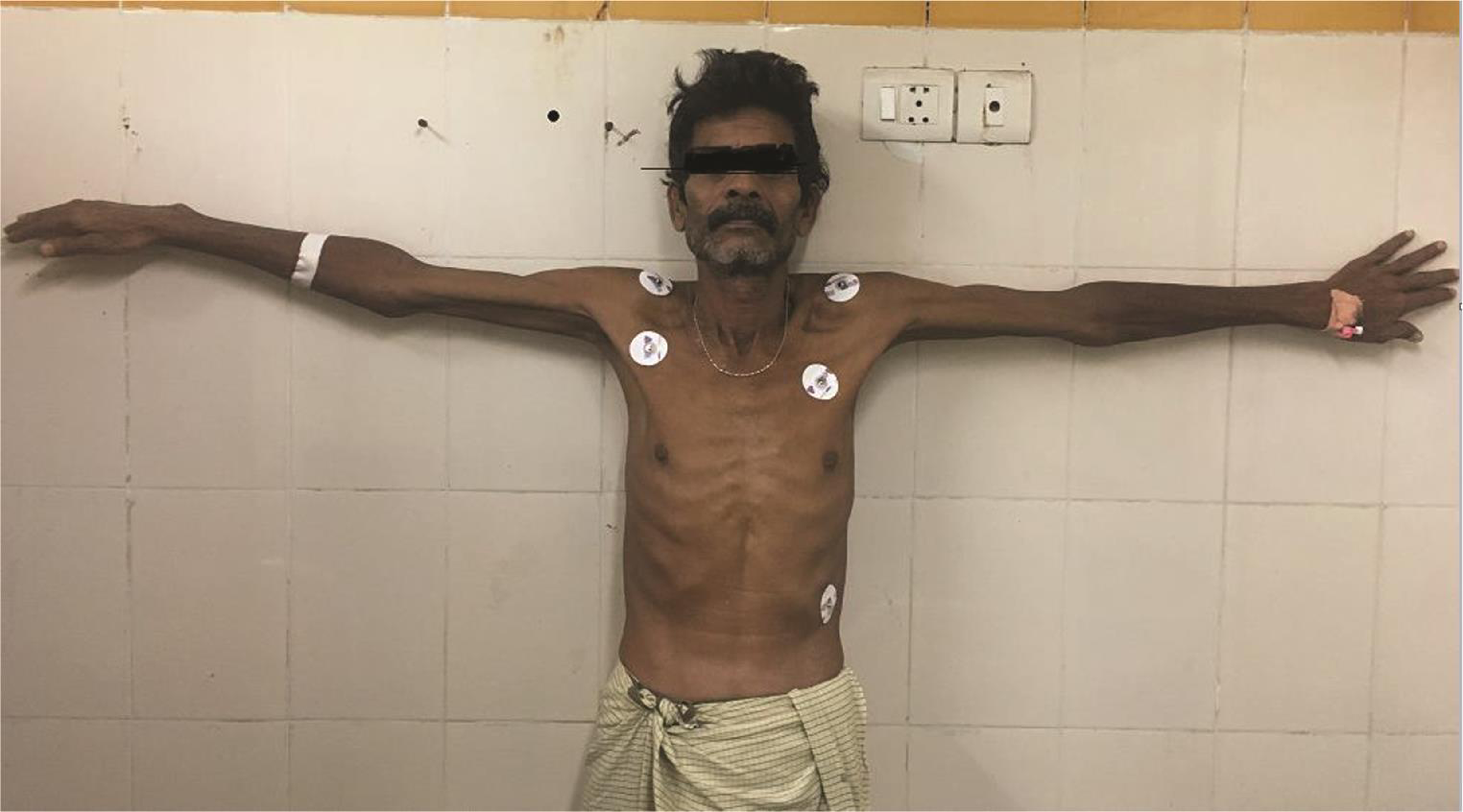
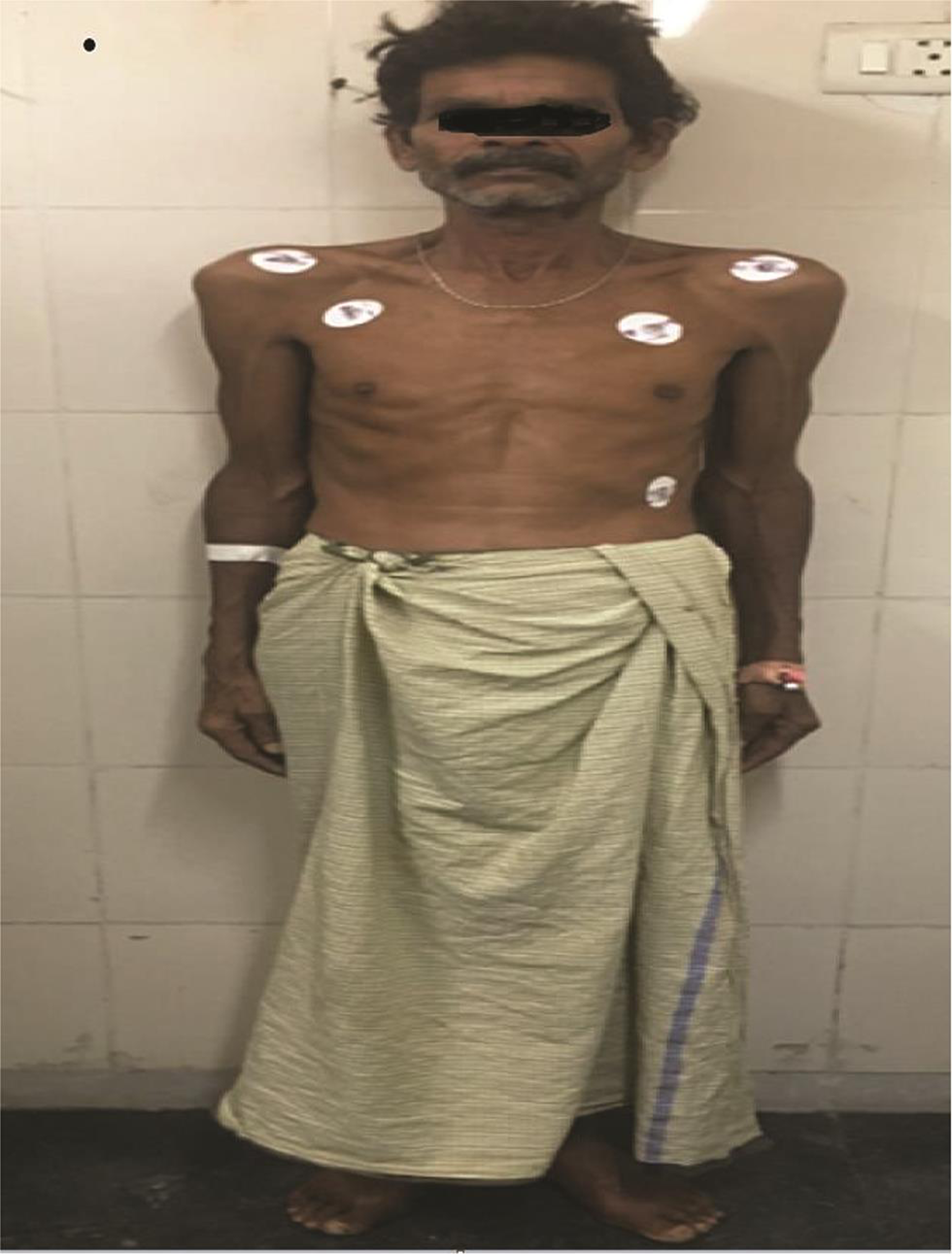
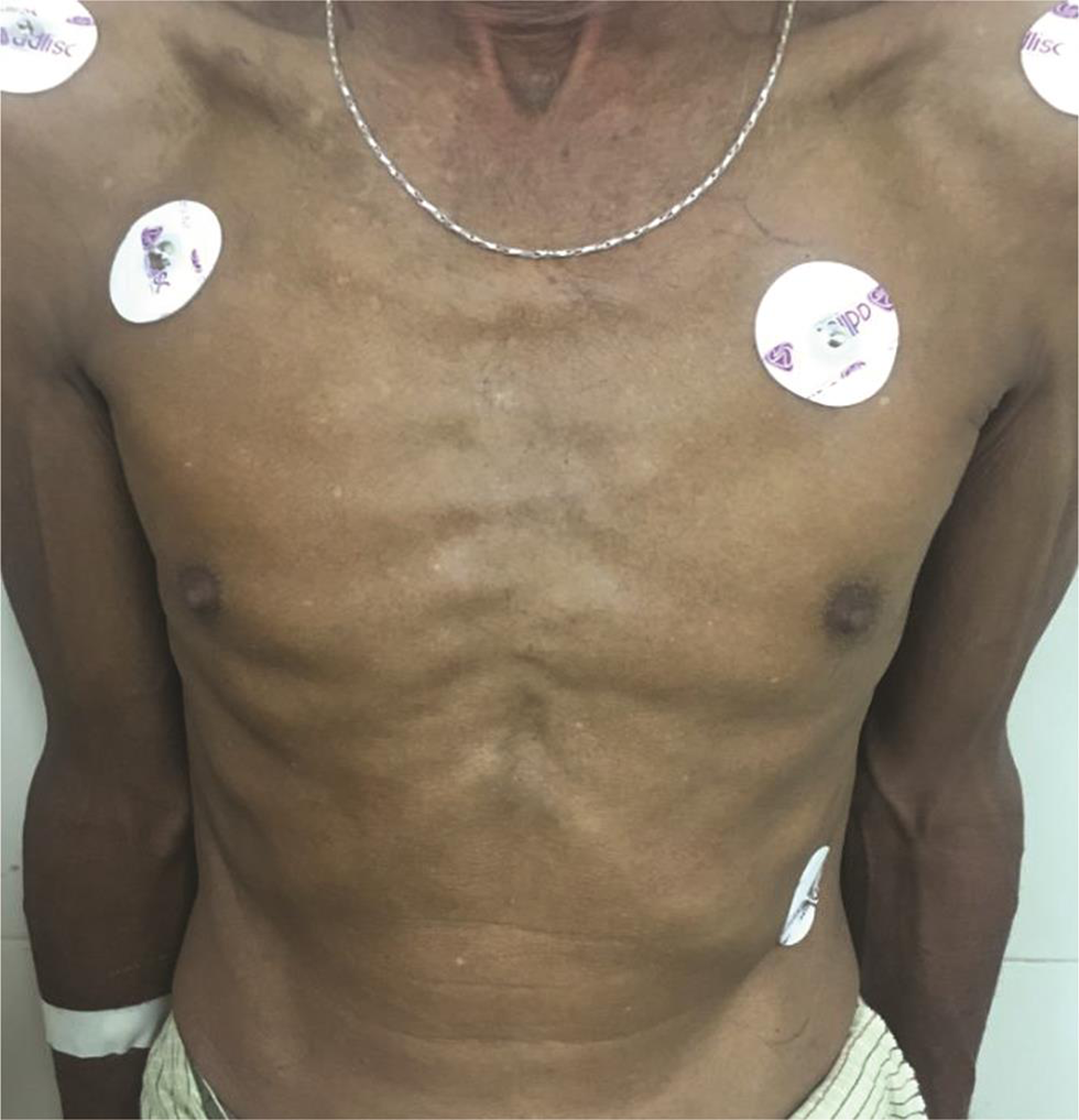
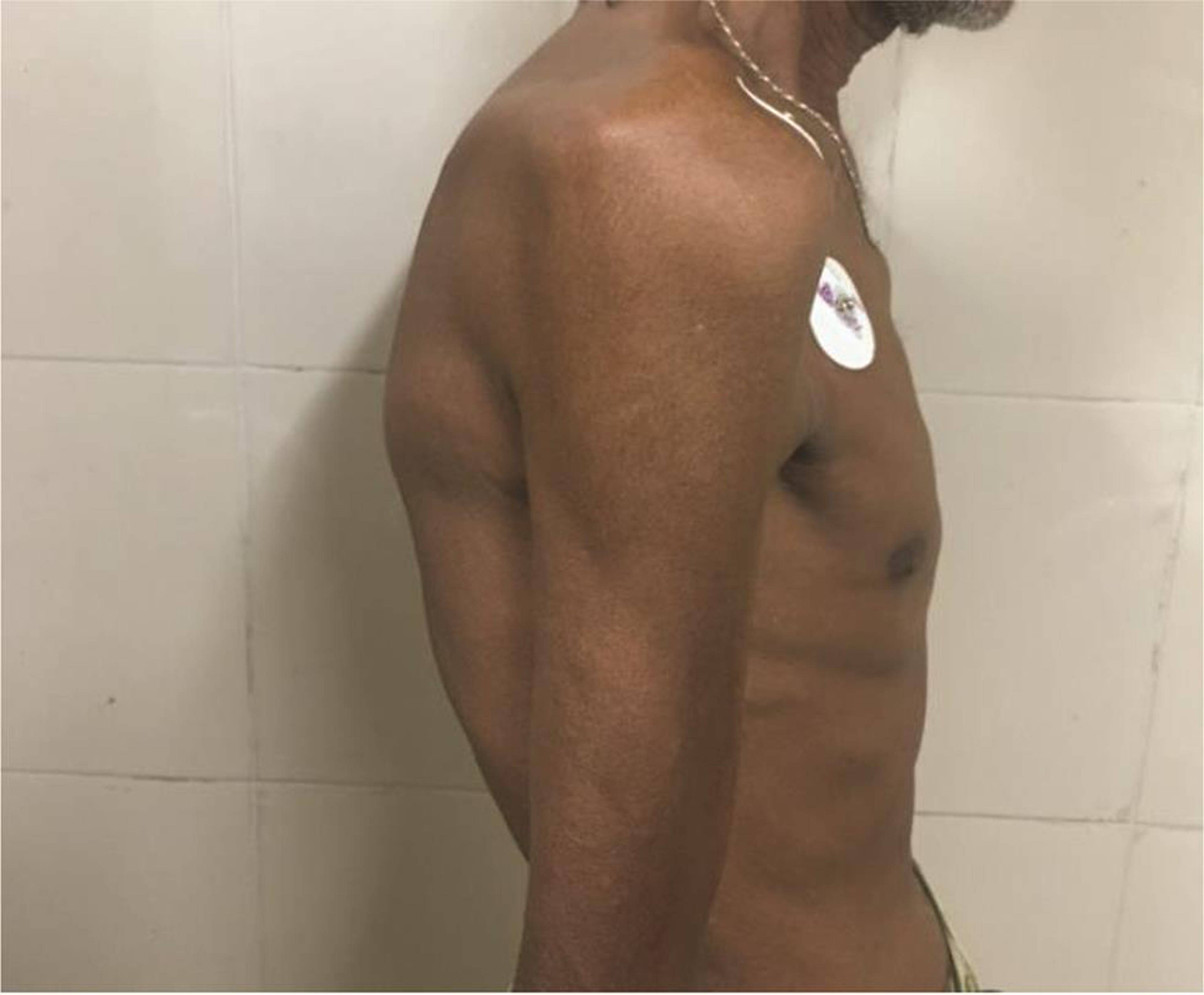
On examination, the patient was alert, cooperative, and well-oriented. The patient appeared too thin and slender (Figs. 1 2) with his body weight measuring less than average for his age and sex. Patient had pectus excavatum (Fig. 3), hunched back (Fig. 4), disproportionately long arms (Fig. 1) and legs (Fig. 2) as compared with the trunk, and his arm span was more than his height by approximately 2 inches (Fig. 1) with an increased floor to pubis measurement/pubis to vertex measurement. In addition to the marfinoid habitus, the patient looked malnourished which needs to be evaluated further. Examination of his hands showed elongated fingers with thickening at the interphalangeal joints. The patient presented with positive wrist sign and a positive Steinberg thumb sign, indicating extreme ligamentous laxity. An examination of the feet revealed that he had flat feet with mild pronation and elongated toes.
-
Fig. 1 Arm span disproportionately longer than the trunk, and a thin and slender body habitus.
Fig. 1 Arm span disproportionately longer than the trunk, and a thin and slender body habitus.
-
Fig. 2 Disproportionately longer arms and legs compared with the trunk.
Fig. 2 Disproportionately longer arms and legs compared with the trunk.
-
Fig. 3 Pectus excavatum chest deformity.
Fig. 3 Pectus excavatum chest deformity.
-
Fig. 4 Hunched back.
Fig. 4 Hunched back.
On respiratory system examination, there was decreased breath sounds in the bilateral basilar areas more on the right side.
On cardiovascular examination, the patient showed distended neck veins, and right ventricle (RV) type of apex 1 cm lateral to the mid clavicular line in the 6th intercostal space. There was a loud S1, loud P2, and 4/6 pansystolic murmur in the left 3rd, 4th and 5th intercostal spaces, increasing in intensity with respiration, which is associated with systolic thrill. There was a murmur which was mid diastolic in timing, best heart at the apex, and ejection systolic murmur best heard on the left side in the 2nd intercostal space just lateral to sternum.
Investigations
Baseline investigation, including complete blood count (CBC), KFT, LFT, prothrombin time (PT/INR), APTT (activated partial thrombin time), were in normal range. His antistreptolysin O (ASO) titers were raised, which supported the diagnosis of rheumatic heart disease.
ECG revealed atrial flutter with fast ventricular rate and variable atrioventricular (AV) conduction.
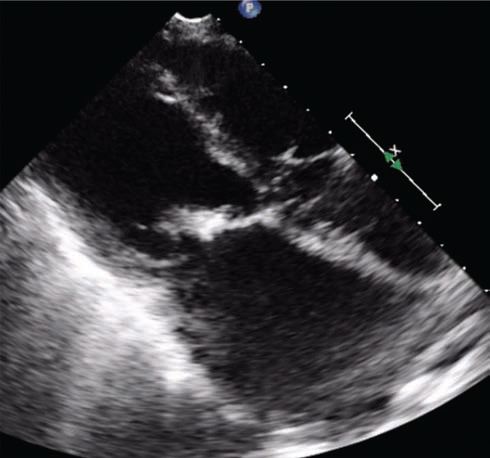
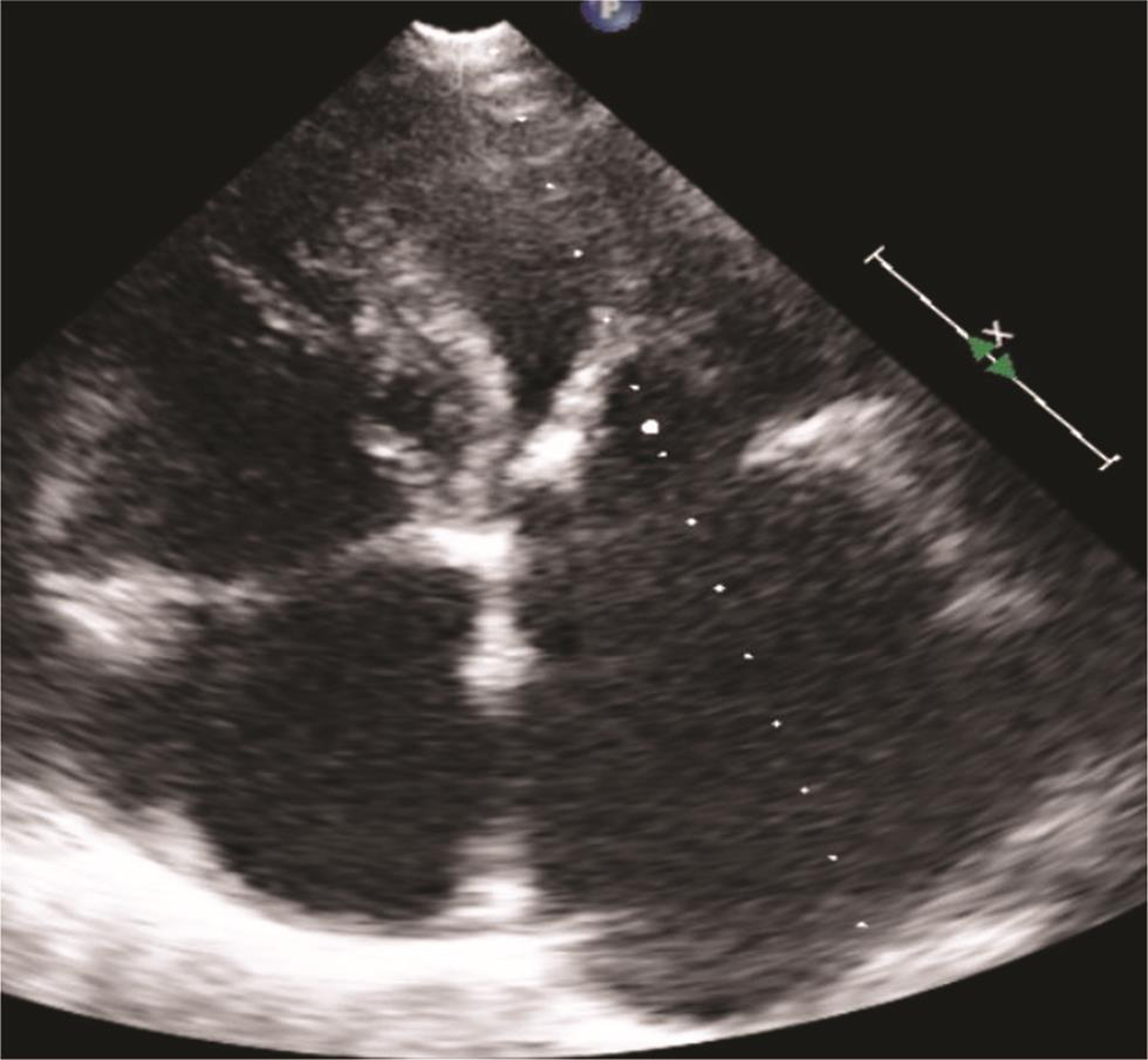
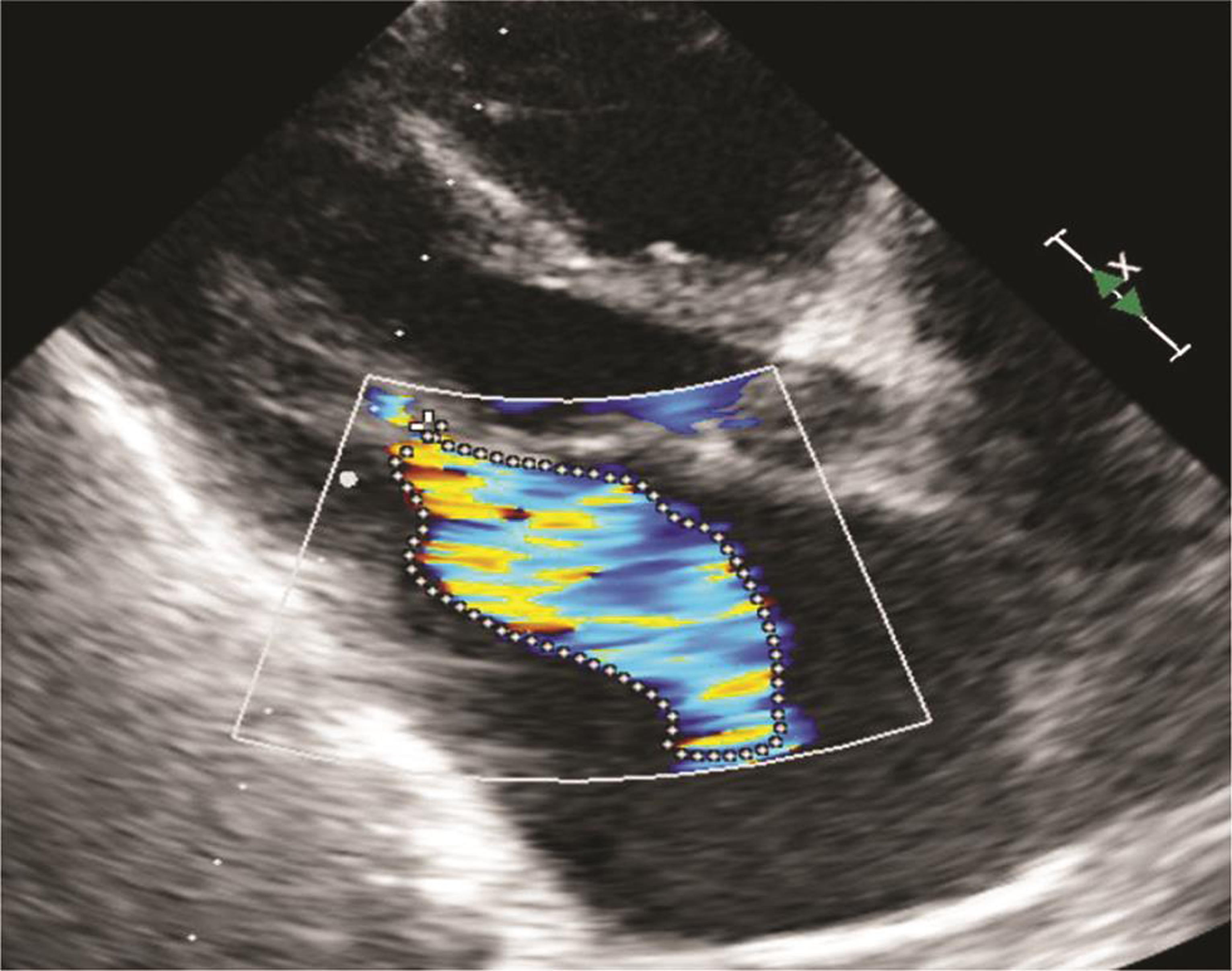
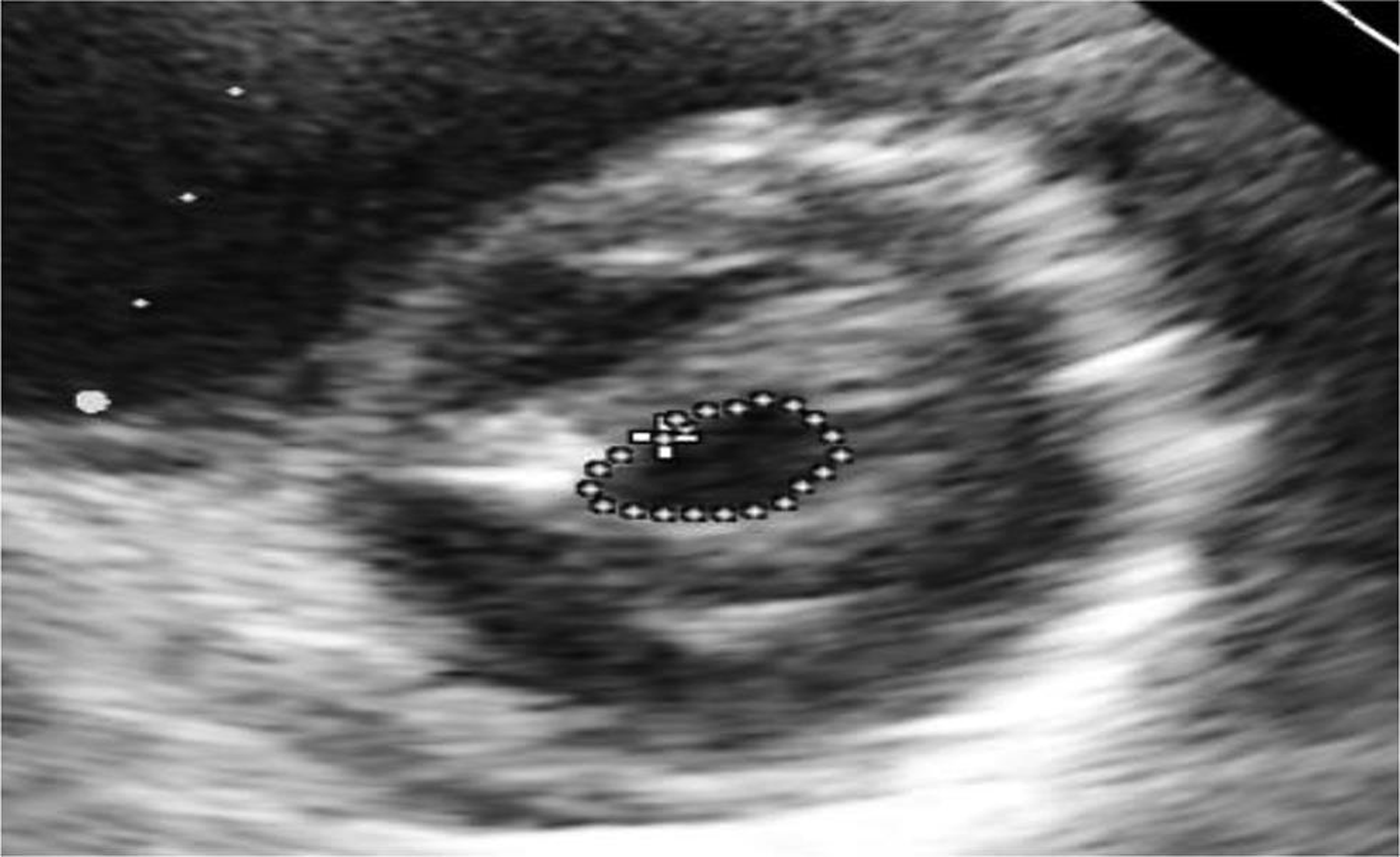
Echocardiography was suggestive of chronic rheumatic heart disease (CRHD), dilated left atrium (Figs. 5 6) dilated right atrium and right ventricle (Fig. 6), severe mitral stenosis (MS) (mitral valve area [MVA] 1.1 cm2) (Fig. 8), moderate mitral regurgitation (MR) (mitral regurgitation jet area [MRJA] 6.0 cm2) (Fig. 7), no aortic stenosis/aortic regurgitation (AS/AR), severe tricuspid regurgitation (TR) (Fig. 9), severe pulmonary arterial hypertension (PAH) (Fig. 10) (right ventricular systolic pressure [RVSP] 100 cm/sec) (Fig. 10), gradient across mitral valve (27/14) (Fig. 11), thickened mitral valve, and paradoxical posterior leaflet motion with decreased anterior mitral leaflet slope (Fig. 12). Normal left ventricular function. No pulmonary embolism (PE)/veg/clot seen.
-
Fig. 5 Echocardiographic image demonstrating dilated left atrium and a thickened and calcified mitral valve (rheumatic mitral valve).
Fig. 5 Echocardiographic image demonstrating dilated left atrium and a thickened and calcified mitral valve (rheumatic mitral valve).
-
Fig. 6 Echocardiographic image: apical four-chamber view showing rheumatic mitral valve, dilated left atrium, right atrium, and right ventricle.
Fig. 6 Echocardiographic image: apical four-chamber view showing rheumatic mitral valve, dilated left atrium, right atrium, and right ventricle.
-
Fig. 7 Echocardiographic image showing moderate mitral regurgitation with an MR jet area of 6.0 cm2. Abbreviation: MR, mitral regurgitation.
Fig. 7 Echocardiographic image showing moderate mitral regurgitation with an MR jet area of 6.0 cm2. Abbreviation: MR, mitral regurgitation.
-
Fig. 8 Echocardiographic image: short axis view showing severe mitral stenosis (MVA–1.1 cm2),which is strong evidence of rheumatic etiology. Abbreviation: MVA, mitral valve area.
Fig. 8 Echocardiographic image: short axis view showing severe mitral stenosis (MVA–1.1 cm2),which is strong evidence of rheumatic etiology. Abbreviation: MVA, mitral valve area.
-
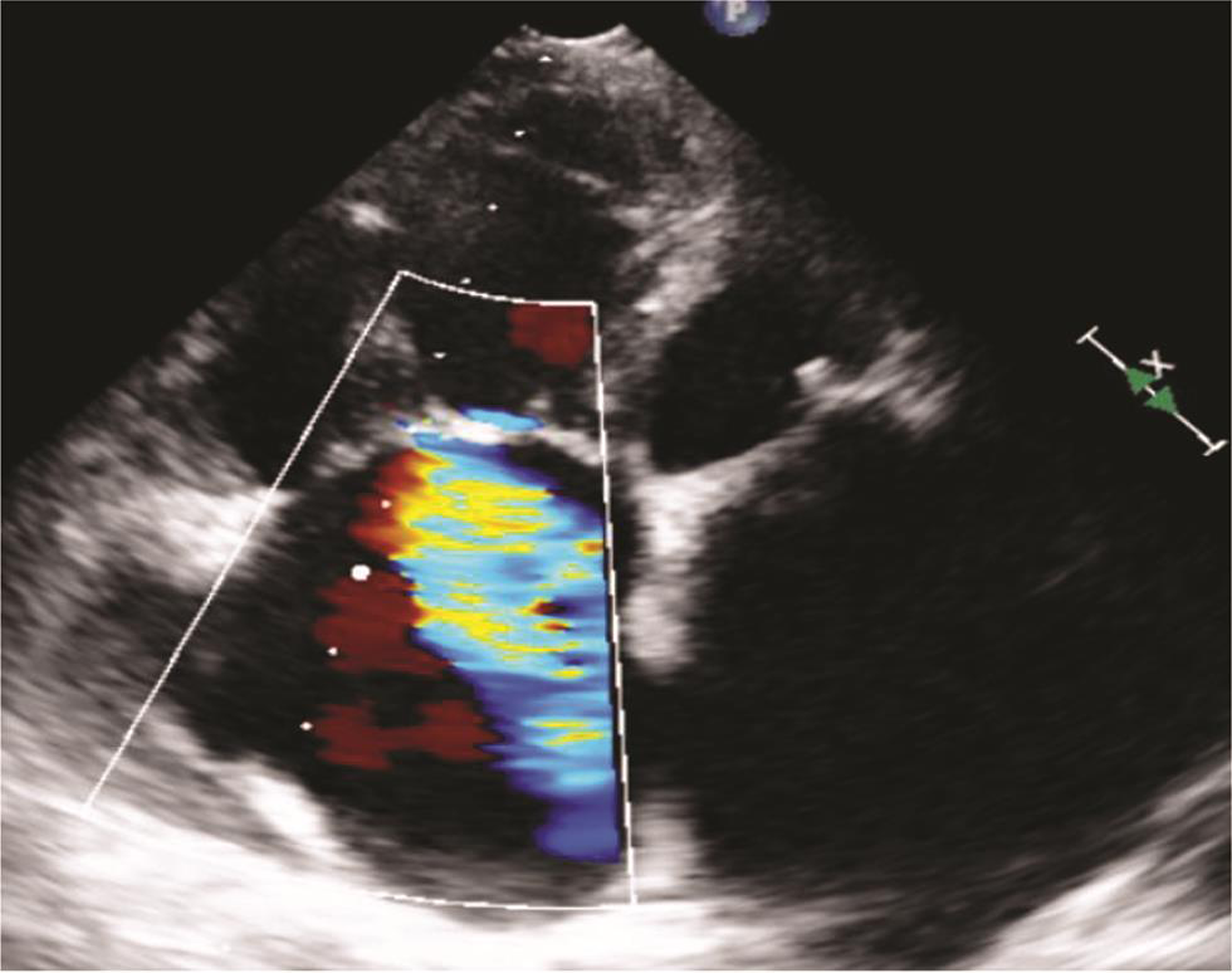
Fig. 9 Echocardiographic image showing severe tricuspid regurgitation.
Fig. 9 Echocardiographic image showing severe tricuspid regurgitation.
-
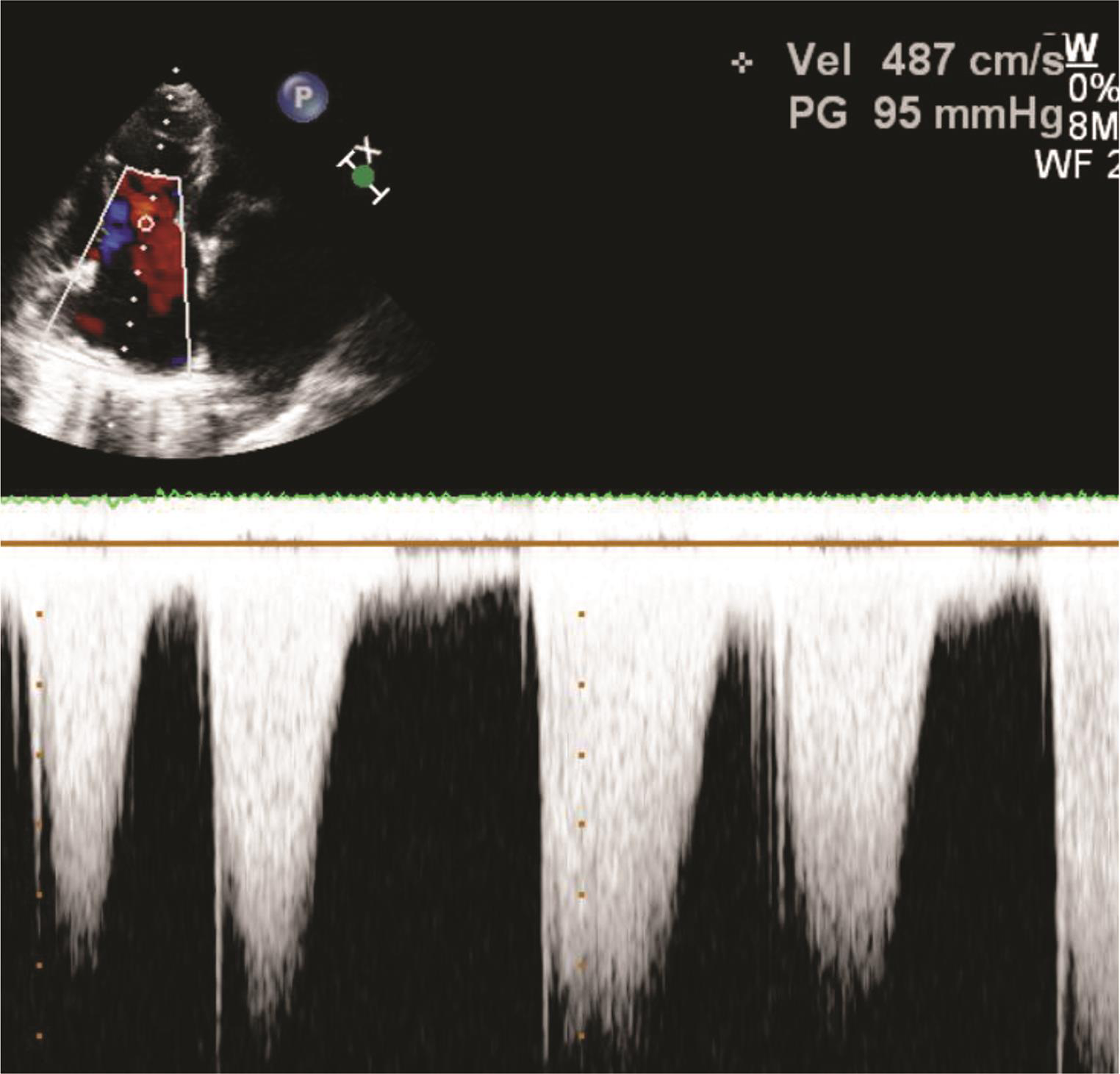
Fig. 10 Echocardiographic image showing severe pulmonary arterial hypertension (RVSP–110 mm Hg). Abbreviation: RVSP, right ventricular systolic pressure.
Fig. 10 Echocardiographic image showing severe pulmonary arterial hypertension (RVSP–110 mm Hg). Abbreviation: RVSP, right ventricular systolic pressure.
-
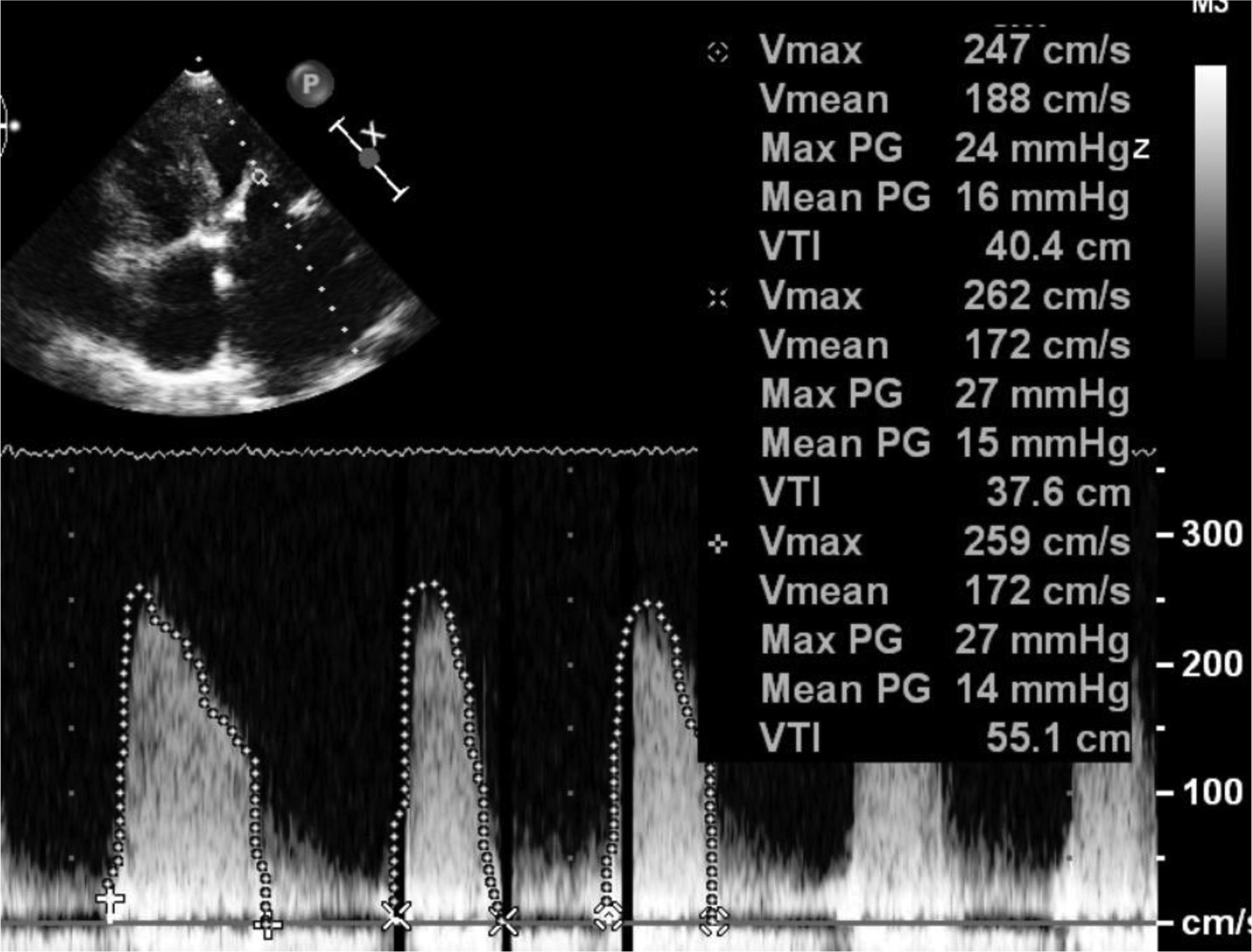
Fig. 11 Echocardiographic image showing increased gradients across mitral valve (MVG–27/14 mm Hg). Abbreviation: MVG, mitral valve gradient.
Fig. 11 Echocardiographic image showing increased gradients across mitral valve (MVG–27/14 mm Hg). Abbreviation: MVG, mitral valve gradient.
-
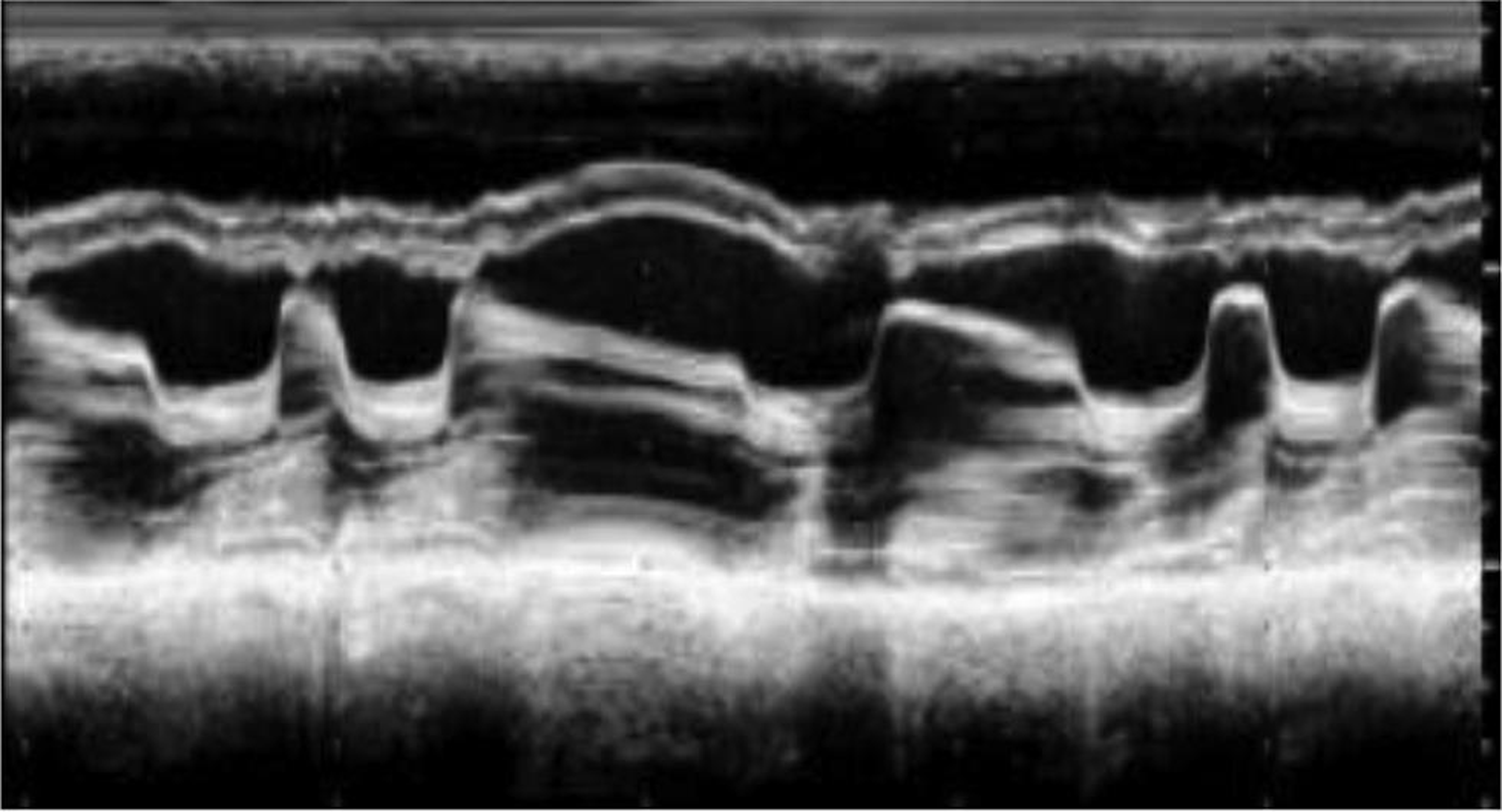
Fig. 12 M mode echocardiography across mitral valve revealing atrial fibrillation, mitral valve thickening, decreased slope of AML, and paradoxical motion of PML. Abbreviations: AML, anterior mitral leaflet; PML, posterior mitral leaflet.
Fig. 12 M mode echocardiography across mitral valve revealing atrial fibrillation, mitral valve thickening, decreased slope of AML, and paradoxical motion of PML. Abbreviations: AML, anterior mitral leaflet; PML, posterior mitral leaflet.
Computed tomography (CT) aortogram was done to rule aortic root involvement. There was no involvement of the aortic root, with the high-resolution computed tomography (HRCT) cuts revealing bilateral pleural effusion, paraseptal emphysema, and calcified granuloma in the right lobe of liver.
Patient underwent coronary angiography (CAG) + catherization to confirm the echocardiographic findings, and evaluate coronaries, so as to plan for surgical intervention. CAG revealed left main coronary artery (LM)–distal 50% stenosis, left anterior descending coronary artery (LAD)–type III, proximal 60 percent stenosis, diagonals normal, proximal left circumflex artery (LCX)–nondominant, ostial LCX 40% disease, obtuse marginal (OM) normal, 2 in number, right coronary artery (RCA) dominant normal, and posterior descending artery (PDA)/postolateral vessel (PLV)–normal. Catheterization data revealed severe pulmonary arterial hypertension (60/20 [40]), increased PCWP–31, and normal left ventricular end diastolic pressures.
Patient was advised mitral valve repair (MVR) + coronary artery bypass grafting (CABG) (left internal mammary artery [LIMA] to LAD graft).
Conclusion
MFS is associated with a variety of cardiovascular manifestations including thoracic artery aneurysms, dissections, and mitral valve prolapse. Our case was unique in the form that patient had associated CRHD, which has been rarely reported (few case reports)13 14 15 previously in cases of MFS.
Conflict of Interest
None declared.
References
- Marfan syndrome and related disorders.the Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill; 1995. p. :4079. In: et al., eds.
- [Google Scholar]
- The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(07):476-485.
- [Google Scholar]
- Color flow and conventional echocardiography of the Marfan syndrome. Echocardiography. 1992;9(06):627-636.
- [Google Scholar]
- Cardiovascular magnetic resonance in Marfan syndrome. J Cardiovasc Magn Reson. 2013;15:33.
- [Google Scholar]
- Mitral valve dysfunction in the Marfan syndrome. Clinical and echocardiographic study of prevalence and natural history. Am J Med. 1983;74(05):797-807.
- [Google Scholar]
- Diagnosis and management of infantile marfan syndrome. Pediatrics. 1990;86(06):888-895.
- [Google Scholar]
- Aggressive primary treatment for poststernotomy acute mediastinitis: our experience with omental- and muscle flaps surgery. Eur J Cardiothorac Surg. 2001;20(04):743-746.
- [Google Scholar]
- Prognosis factors in probands with an FBN1 mutation diagnosed before the age of 1 year. Pediatr Res. 2011;69(03):265-270.
- [Google Scholar]
- Left ventricular systolic dysfunction in asymptomatic Marfan syndrome patients is related to the severity of gene mutation: insights from the novel three dimensional speckle tracking echocardiography. PLoS One. 2015;10(04):e0124112.
- [Google Scholar]
- A patient with Marfan’s syndrome presented with severe rheumatic mitral stenosis and successfully treated with percutaneous transmitral balloon commissurotomy - Report of first case. J Cardiovasc Dis Res. 2013;4(04):257-259.
- [Google Scholar]
- Rheumatic heart disease in a patient with Marfan’s syndrome. International Journal of Case Reports and Images 2019 (e-pub ahead of print)
- [CrossRef] [Google Scholar]
- Severe rheumatic mitral stenosis in marfanoid young man successfully managed with percutaneous mitral balloon valvotomy. Journal of Case Reports. 2016;6(03):338-341.
- [Google Scholar]







