Translate this page into:
An Unusual Presentation of Primary Anti-phospholipid Antibody Syndrome as Acute Coronary Syndrome in Young – A Case Report
*Corresponding author: Siddhartha Pandey, Department of Cardiology, Srirama Chandra Bhanja (SCB) Medical College, Cuttack, Odisha, India. sidarine@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mohanty N, Dash BK, Routray S, Pandey S. An Unusual Presentation of Primary Anti-phospholipid Antibody Syndrome as Acute Coronary Syndrome in Young – A Case Report. Indian J Cardiovasc Dis Women. 2024;9:35-9. doi: 10.25259/IJCDW_54_2023
Abstract
A case report of a 32-year-old female patient without any traditional risk factors presented with angina to our hospital within 6 h. Her electrocardiogram (ECG) showed extensive anterior wall -ST elevation myocardial infarction (MI). She was treated with thrombolytic therapy in the form of tenecteplase and was given standard treatment for acute coronary syndrome (ACS). Her history revealed three recurrent pregnancy losses and a history of preeclampsia. Echocardiography showed regional wall motion abnormalities of the mid, distal, apex, and anterior wall with severe left ventricular systolic dysfunction. Routine blood investigations showed elevated total leukocyte count with neutrophilic predominance. With a history of recurrent pregnancy loss, she was investigated for anti-phospholipid anti-phospholipid antibody syndrome (APS) and was found to have positive lupus anticoagulant 1. She underwent coronary angiography (CAG) which showed a left main shaft 40% stenosis with an ulcerative lesion containing thrombus in the ostioproximal to mid-left anterior descending artery (LAD), other coronary arteries were normal. She was managed conservatively with glycoprotein IIb/IIIa inhibitor (Tirofiban) and switched to oral anticoagulation with Vitamin K antagonist (VKA-Acenocoumarin) along with dual-antiplatelet therapy.
Keywords
Acute coronary syndrome
Anti-phospholipid antibody syndrome
Vitamin K antagonist
INTRODUCTION
Antiphospholipid syndrome (APS) is a multisystem autoimmune disease most commonly associated with recurrent arterial and venous thromboembolism and recurrent fetal loss. Cardiac involvement is immune mediated and or by thrombotic mechanisms. However, the cardiovascular risk in patients with primary APS Primary anti-phospholipid antibody syndrome (PAPS) compared with lupus-related APS is yet to be established. Cardiac symptoms of APS include valve abnormalities (thickening and vegetation), coronary artery disease (CAD), myocardial dysfunction, pulmonary hypertension, and intracardiac thrombi.[1]
The association between APS and acute myocardial infarction (AMI) is more frequent in women. There is some evidence that ∼2.8–5.5% of cases of AMI in young individuals are secondary to APS.[2] Accelerated atherosclerosis of the coronary arteries as well as microvascular injury or coronary thromboembolism may lead to ischemia and is one of the major causes of myocardial infarction (MI) in the young.[3] The thrombotic manifestations in patients with APS are described by a “two-hit model,” suggesting that a “second hit” or “trigger” is needed to push the hemostatic balance toward clot formation. These triggers include environmental (e.g., infection, surgery, and immobilization), inflammatory (e.g., autoimmune diseases), or other non-immunological procoagulant factors (e.g., oral contraceptive use).[4] Very few cases have been reported with primary APS presenting as acute coronary syndrome (ACS).
CASE REPORT
A 32-year-old female presented with typical retrosternal chest pain to the Department of Cardiology within 6 h with no traditional risk factors. There was a history of recurrent pregnancy loss and pre-eclampsia at 32 weeks, 28 weeks, and 24 weeks of gestation in 2015, 2017, and 2021, respectively. Electrocardiogram (ECG) showed extensive anterior wall ST-elevation MI [Figure 1]. Physical examination on admission did not reveal any abnormality. She was given treatment as per standard ACS protocol and was given fibrinolytic therapy with tenecteplase. Routine blood investigations revealed elevated total leukocyte counts (TLC-41,000/mm3) and activated partial thromboplastin time (aPTT)-46.7 s (control 28.5 s) [Table 1] and lupus anticoagulant 1 (LA1) was moderately positive [Table 1]. Given increased TLC count, the patient was started on injection meropenem 1 g I.V. 8 hourly. On day 2, the patient complained of shortness of breath New York Heart Association class IV; on examination, blood pressure was 80/60 mm of Hg and there was bilateral diffuse crepitations and left ventricular (LV) S3 suggestive of pulmonary edema. The patient was started on inotropes and was given diuretics with vital monitoring. A bedside 2D echo evaluation was done which showed regional wall motion abnormality in the anterior wall with LV dysfunction (ejection fraction [EF] = 30–35%) and chest X-ray showed pulmonary edema [Figure 2]. The patient became symptomatically better, blood pressure normalized, and her total leukocyte count (TLC) counts came to 18,000/mm3. Hematological tests were performed in view of thrombotic disorders and her activated partial thromboplastin time (aPTT) was 46.7, and LA1 was 62.4 s (reference: 28–48 s) which was moderately positive using diluted russel viper venom time (dRVVT) method, serum homocysteine was 8 μmol/L (reference: 5–13 μmol/L), and lipoprotein a was 18 ng/dL (reference: <38 ng/dL. Her past records showed positive immunoglobulin M (IgM) anticardiolipin antibody-32.43 MPL (Reference <15 MPL units). On stabilization, a repeat 2D echo was done which showed severe left ventricular (LV) systolic dysfunction (EF-30–35%) and the presence of thrombus in the LV apex [Figure 3]. A lower limb Doppler was done which was normal. On the subsequent day, she underwent coronary angiography (CAG), which revealed a left main shaft 40% stenosis and an ulcerative lesion containing thrombus in ostioproximal to the mid left anterior descending artery (LAD) with TIMI III flow, left circumflex and right coronary artery was normal [Figure 4]. She was managed conservatively with glycoprotein IIb/IIIa inhibitor given for 24 h. On discharge, a repeat echocardiographic evaluation was done which showed an ejection fraction (EF) of 40%. The patient was started on oral anticoagulation (Vitamin K antagonist [VKA]-acenocoumarin). Her final diagnosis was extensive anterior wall ST elevation MI with severe LV systolic dysfunction with LV apical thrombus possible etiology primary antiphospholipid antibody syndrome (APS), accelerated atherosclerosis with thrombus in coronary arteries. She was discharged on dual antiplatelet therapy (DAPT) and Vitamin k antagonist (VKA) with international normalized ratio - 2.9 and is planned for myocardial viability testing and subsequent revascularization. The patient was followed up after 3 months and the antiphospholipid antibody panel was repeated and was negative suggestive of transient elevation.
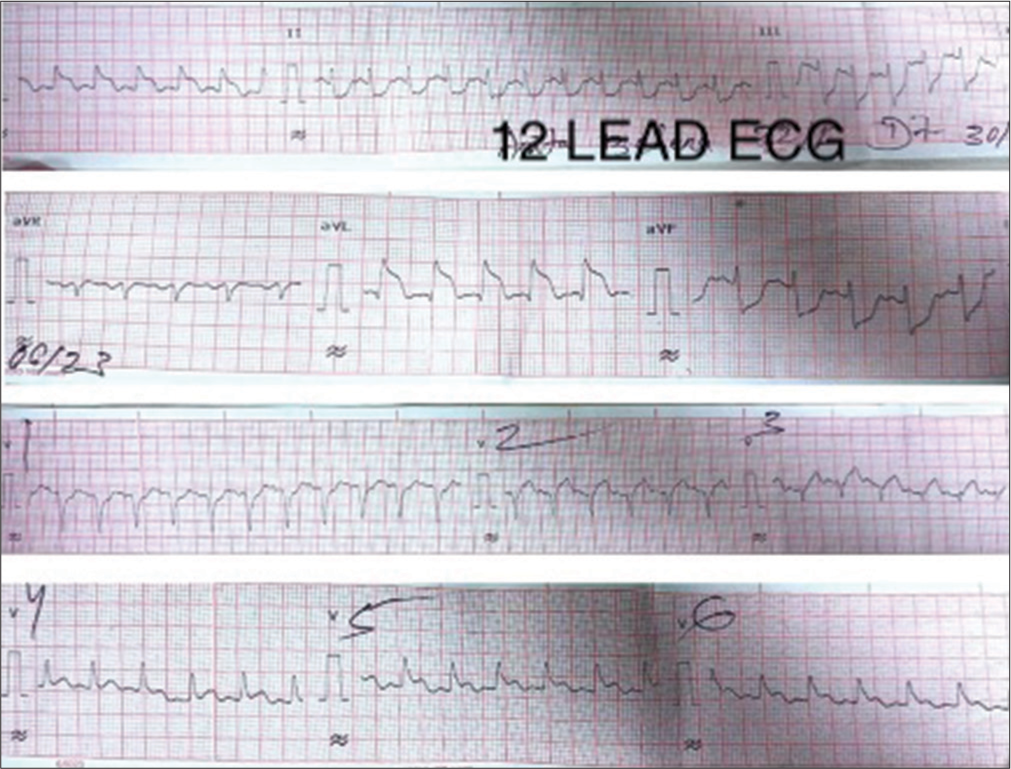
- 12 Lead ECG showing extensive ST-segment elevation myocardial infarction. (ECG: Electrocardiogram).
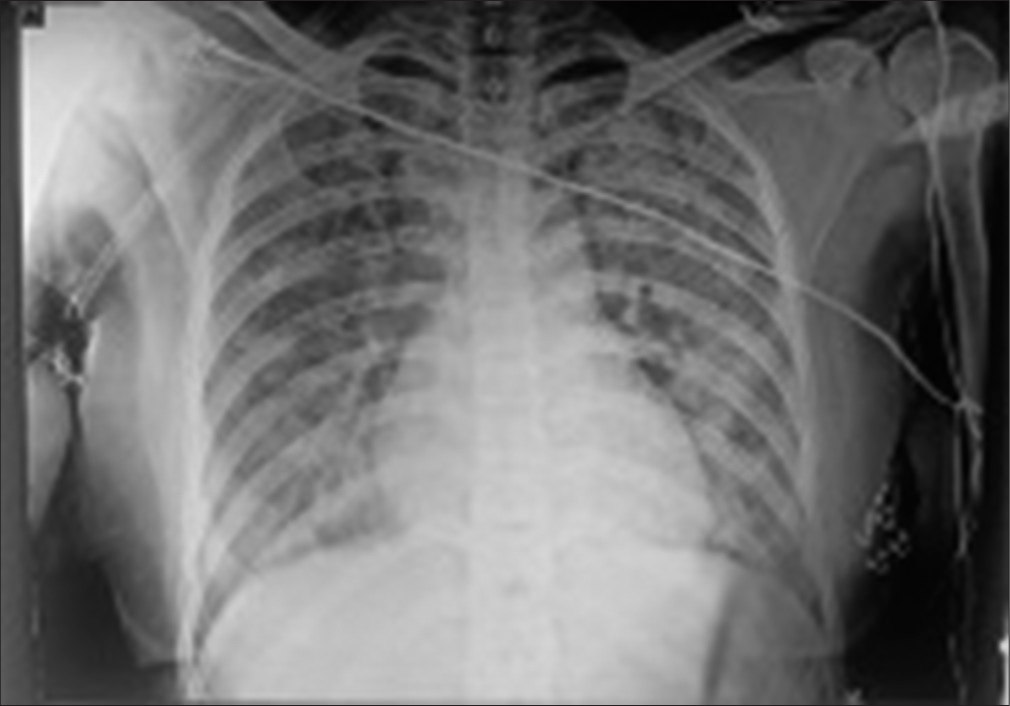
- Chest X-ray anterior-posterior view.

- Thrombus in the left ventricular apex.
| D1 | D2 | D3 | D4 | D7 | |
|---|---|---|---|---|---|
| HB (g/dL) | 17 | 13 | 12 | 12.2 | |
| TLC (×103 mm3) | 41 | 28 | 18 | 16.4 | |
| Platelet (Lakhs) | 466 | 419 | 369 | 365 | |
| FBS/PPBS (mg/dL) | 206/233 | 134/181 | |||
| S. urea (mg/dL) | 58 | 81 | 66 | ||
| S. creatinine (mg/dL) | 1.8 | 1.4 | 1.0 | ||
| HBA1C | 5.5 | ||||
| PT/INR | 18/1.4 | 58/2.9 | |||
| APTT (control-28.5 s) | 46.7 | ||||
| Procalcitonin (ng/mL) | 4.12 | 0.19 | |||
| CRP (Q)-mg/dL | |||||
| D-DIMER (0–500) ng/mL | 424 | ||||
| RA factor | |||||
| Blood C/S | No growth | ||||
| LA | Reference | ||||
| LA 1 | 62.4 s | 28–48 | |||
| LA 2 | 38.1 s | 28–40 | |||
| Ratio (LA1/LA2) | 1.64 | <1.2 | |||
| ANA Hep 2 | Negative | ||||
| Anti-phospholipid antibody (IgG) | 0.16 (negative) | ||||
| Anticardiolipin antibody (IgG) | <9.37 (IgG phospholipid units) | ||||
| S. homocysteine | 8 μmol/L | Reference 5–13 μmol/L |
|||
| Lipoprotein (a) | 18ng/dL | Reference 38 ng/dL |
|||
| Lipid profile (mg/dL) | Reference | ||||
| T. cholesterol | 119 | <200 mg/dL | |||
| S. triglycerides | 112 | <180 mg/dL | |||
| HDL | 32 | ||||
| LDL | 64 | <130 mg/dL | |||
| VLDL | 22 |
HB: Hemoglobin, TLC: Total leukocyte count, FBS/PBBS: Fasting blood sugar/post-prandial blood sugar, S. urea: Serum urea, S. creatinine: Serum creatinine, HBA1C: Hemoglobin A1C, PT: Prothrombin Time, INR: International Normalised Ratio APTT: Activated partial thromboplastin time, CRP: C-reactive protein, RA: Rheumatoid arthritis, C/S: Culture and sensitivity, LA: Lupus anticoagulant, ANA: Antinuclear antibody, IgG: Immunoglobulin G, S. homocysteine: Serum homocysteine, T. cholesterol: Total cholesterol, LDL: Low-density lipoprotein, VLDL: Very-low-density lipoprotein, HDL: High density lipoprotein. , D1: Day 1, D2: Day 2, D3: Day 3, D4: Day 4, D7: Day 7, GPL: IgG phospholipid units
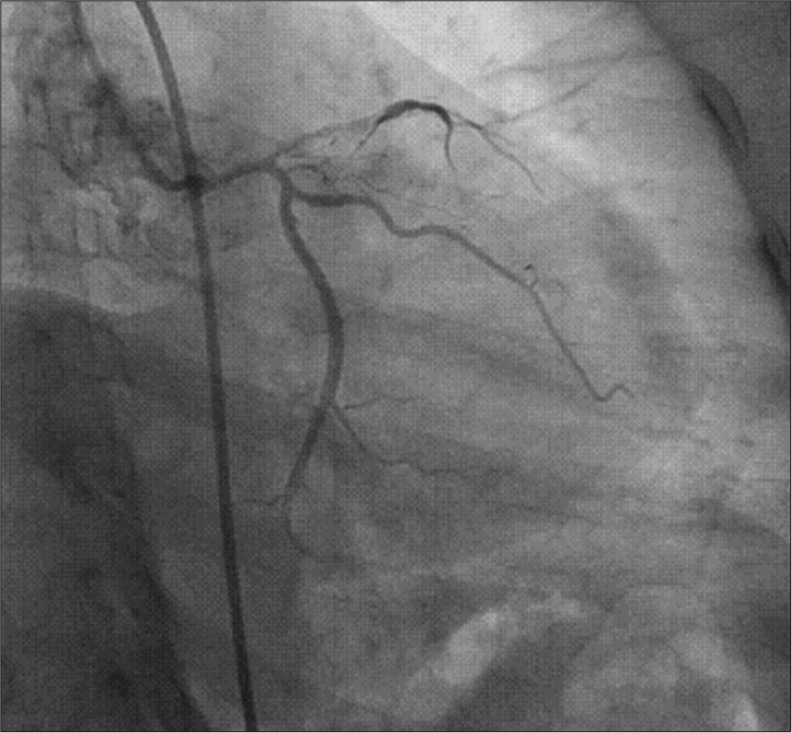
- Diffuse ulcerative lesion containing thrombus from ostioproximal to mid left anterior descending artery.
DISCUSSION
Anti-phospholipid syndrome (APS) is most commonly associated with recurrent arterial and venous thromboembolism and recurrent fetal loss. MI in young adults is unusual, but some cases have been reported in patients with APS. Abid et al reported the observations of 2 young adults (one woman and one man), with AMI. A 30-year-old woman with a history of recurrent spontaneous abortion presented with acute anterior MI complicated by cardiogenic shock. Coronary angiography showed thrombosis and stenosis in the proximal left anterior descending (LAD) and underwent percutaneous transluminal coronary angioplasty (PTCA). Lupus anticoagulant was negative. Anticardiolipin IgM and IgG, anti-2GPI antibodies, and anticardiolipin antibody (ACL) were positive. Second case a 30-year-old Caucasian man presented with bilateral acute ischemia of lower limbs. Arterial pressure was normal. The electrocardiogram showed a QS pattern in precordial leads. Echocardiography revealed an apical large thrombus associated with a thinning LV wall with severe LV systolic dysfunction. Hematological tests showed normal levels of C, S, and ATIII protein but positive lupus anticoagulant and anticardiolipin antibodies. APS syndrome was considered primary in the above two cases.[5] Yaghoubi et al., studied 133 patients with premature coronary artery disease (CAD). In their study, 18 patients were confirmed to have APS (13.53%). The results showed that there was an association between the risk of developing premature CAD and APS and it should be considered an important etiology for patients presenting with ACS in the young population.[6] Takeuchi et al. described a case of a 20-year-old man with severe chest pain hospitalized for acute MI. Coronary angiography revealed total obstruction of his right coronary artery, which was successfully recanalized by direct PTCA and diffuse thrombi in the left coronary artery that was not recanalized by prourokinase. Anticoagulant therapy was given after PTCA. The aPTT was 62.6 s and plasma anticardiolipin IgG antibodies were high (3.8 and 2.7) in repeated examinations. They concluded that primary antiphospholipid syndrome should be considered as a cause of acute MI in young adults, and PTCA with anticoagulant treatment is effective for the initial treatment of the syndrome.[7] In our case, a young female with a history of recurrent pregnancy loss, without any traditional risk factors for CAD presented with extensive anterior wall ST elevation MI. Her hematological test for thrombotic disorders revealed positive LA1. A 2D-echocardiography showed the presence of thrombus in LV apex and coronary artery disease (CAD) revealed the left main shaft at 40% and an ulcerative lesion with thrombus in ostioproximal to mid LAD. APS patients with ST elevation myocardial infarction should undergo revascularization, if feasible, usually associated with thrombus aspiration, and in selective cases stent implantation in the culprit lesion. Triple antithrombotic therapy with short-term dual antiplatelet therapy (DAPT) and long-term anticoagulant therapy is recommended.[8] The management in such cases differs from other ACS patients as these patients with APS require oral anticoagulation along with antiplatelet therapy. Oral Vitamin K antagonist is the agent of choice for APS patients and new oral anticoagulants are not recommend ed.[9-11]
Limitations
Intravascular coronary imaging was not available which could have given a better understanding of the lesion morphology and would have helped with further plan of management.
CONCLUSION
Patients presenting with ACS at a young age should be evaluated for anti-phospholipid antibody syndrome along with other thrombotic disorders.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as the patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cardiac Manifestations of Antiphospholipid Syndrome with Focus on its Primary Form. Front Immunol. 2019;10:941.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac manifestations in antiphospholipid syndrome In: Khamashta MA, ed. Hughes Syndrome (2nd ed). Singapore: Springer; 2006. p. :13-41.
- [CrossRef] [Google Scholar]
- Acute Myocardial Infarction and Antiphospholipid Antibody Syndrome: A Systematic Review. Coron Artery Dis. 2017;28:332-5.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-Coated Balloons for Small Coronary Artery Disease (BASKET-SMALL 2): An Open-Label Randomised Non-Inferiority Trial. Lancet. 2018;392:849-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Myocardial Infarction in Young Adults with Antiphospholipid Syndrome: Report of Two Cases and Literature Review. Pan Afr Med J. 2011;8:13.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of Serum Levels of Vitronectin, Malondialdehyde and Hs-CRP with Disease Severity in Coronary Artery Disease. J Cardiovasc Thorac Res. 2015;7:113-7.
- [CrossRef] [PubMed] [Google Scholar]
- Primary Antiphospholipid Syndrome with Acute Myocardial Infarction Recanalised by PTCA. Heart. 1998;79:96-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Myocardial Infarction Due to APS-Case Report and Review of the Literature. Curr Probl Cardiol. 2021;46:100552.
- [CrossRef] [PubMed] [Google Scholar]
- Addendum to British Society for Haematology Guidelines on Investigation and Management of Antiphospholipid Syndrome, 2012 (Br J. Haematol. 2012;157:47-58): Use of Direct Acting Oral Anticoagulants. Br J Haematol. 2020;189:212-5.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Direct Oral Anticoagulants in Patients with Thrombotic Antiphospholipid Syndrome: Guidance from The Scientific and Standardization Committee of The International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-37.
- [CrossRef] [PubMed] [Google Scholar]
- EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann Rheum Dis. 2019;78:1296-304.
- [CrossRef] [PubMed] [Google Scholar]







