Translate this page into:
Venous Intervention
*Corresponding author: Dr. Rajeev Bhardwaj, Professor and Head, Department of Cardiology, Maharishi Markandeshwar Institute of Medical Sciences and Research, Ambala, Haryana, India. rajeevbhardwaj_dr@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Bhardwaj R, Bhambhani A, Patibandla S, Mirza M, Aggrawal G. Venous intervention. Indian J Cardiovasc Dis Women 2022;7:220-7.
Abstract
Any overview of the treatment of venous disease should begin with a brief examination of its history. From the first rudimentary attempt at venous thrombectomy in the early 1920s to the evolution of percutaneous and mechanical thrombectomy and endovascular stents in the 21st century. It is the aim of this review to provide a comprehensive summary of the state of the art of venous disease treatment at the turn of the new century.
Keywords
Venous intervention
Thrombosis
DVT
INTRODUCTION
Venous intervention
There has been a steady advancement in the treatment of venous diseases from the early 1920s from basic attempts on venous thrombectomy to the evolution of intravenous and catheter directed thrombolysis (CDT) for venous thrombosis and then to the placement of endovascular stents. In this review, attempts have been made to discuss the advanced interventional therapy for the management of venous diseases in the present era.
Options to reduce thrombus burden
Options available to reduce the thrombus burden include mechanical thrombectomy (MT), CDT, or combination of pharmacological and mechanical thrombectomy (PMT).
CDT selectively administers the thrombolytic agent into the thrombus and is usually the first line of the treatment.[1-3]
LOWER VEIN DEEP VENOUS THROMBOSIS (DVT)
Standard treatment of lower limb DVT consists of intravenous (l/V) heparin therapy followed by oral anticoagulants for 3–6 months. However, in few cases, thrombolysis may be required.
Catheter-directed pharmacologic thrombolysis
It is an important advancement for iliocaval venous occlusion and proximal DVT. Main benefit is direct delivery of the drug through a catheter into the thrombus at that site. This therapy is successful most likely during an acute event (<14 days old) and is less effective in chronic condition (>4 weeks old).[4] The thrombolytic agents used are streptokinase, reteplase, tenecteplase, alteplase, and urokinase. However, none has specific FDA approval for their use in DVT. However, small studies show that CDT may reduce post-thrombotic syndrome (PTS). National Venous Thrombolysis Registry shows a strong point of a relationship between the degree of thrombolysis and patency of the vessel. In this multicenter study, the use of CDT with agent urokinase in 303 limbs of 287 patients was evaluated. In this study, iliofemoral DVT was most common and 25% of the patients had isolated femoropopliteal DVT. On venography, complete lysis of the clot defined as grade III lysis was seen in only 31% of cases and partial lysis of the clot, that is, >50% of resolution of clot (Grade II lysis) was seen in 52% of the cases. Moreover, venous patency also showed improved valvular function. In this study, 72% of patients who had optimal lysis of the clot maintained normal function of the valve but in 62% of patients who had incomplete lysis of the clot had valvular incompetence. Major bleeding was seen in 11% of patients and was seen at venous access sites.[5]
In patients presenting with symptoms of massive swelling of limbs associated with severe pain, intervention helps reduce mortality and prevention to venous gangrene. In the CAVNET study, in CDT group, the incidence of PTS was reduced to 14.4%, and venous patency rate at 6 months was 65.9% versus 47.4% in control group (P = 0.012).[6] However, in large studies, it was not seen. In the ATTRACT study which was a randomized and multicenter trial, at 2 years, there was no reduction of overall PTS (48.0% vs. 47.4%), but there was a significant overall difference of 6% decline in moderate to severe degree of PTS (P = 0.04).[7]
Thrombolysis is started with an initial bolus dose of 2–4 mg tPA. For pulse spray CDT with rtPA, weight-based dosing is started at the rate of 0.01 mg/kg/h with maximum of 1.0 mg/h.[8] Low-dose heparin is administered concurrently (500 units/h) to prevent thrombosis of the access sheath.
Dose of urokinase is started as infusion of 1−2.5 lac IU of urokinase over 15 min and followed by 5–10 lac IU in 24 h, for 48–72 h.
[Table 1] shows the summary of trials of CDT.
| Study (year) | Design | Limbs treated | Pathology | Arms | Agent | Short term patency | Long term patency |
|---|---|---|---|---|---|---|---|
| Bjarnason et al. (1977)[32] |
Institution series | 87 | Acute iliofemoral DVT | CDT(87)±PTA ±Stent±PMT |
Urokinase | Immediate 69 (79%) iliac, 86%, femoral 63% | 1 y: iliac 63% primary, 78% secondary |
| Mewissen et al. (1993)[5] |
National Registry data | 303 | Acute and chronic suprapopliteal | CDT | Urokinase | Immediate grade III in 96 (31%), II in 17%, 162(52%), I in 54 | 1 y: 181 (60%) |
| Rao et al. (2009)[33] |
Institution series | 43 | Symptomatic iliofemoral DVT (19, >14 days) | CDT+PMT | r-tPA | Immediate: grade III, III lysis in 41 (95%) | Not reported |
| Baekgaard et al(2010)[34] |
Institution series | 103 | DVT<14 days, open distal, popliteal vein | CDT+stockings (130)+stent (57) |
r-tPA | 1 week: 95/103 (92%) | 6 y: 84 (82%) mean follow-up 50 mo |
| Enden et al. (2012)[6] |
Randomized controlled trial | 209 | Acute iliofemoral DVT | CDT±angioplasty ±stent versus anticoagulation alone |
r-tPA | 44.4% with PTS in CDT versus 55.6% in control group at 2 y follow up |
DVT: Deep venous thrombosis, CDT: Catheter directed thrombolysis, PMT: Pharmacological and mechanical thrombectomy
After CDT, the thrombus will become soft and can be easily aspirated through a larger catheter with mechanical suction, using a syringe. In recent years, the AngioJet Rheolytic catheter System (MEDRAD Interventional, Minneapolis, MN) and an ultrasound-emitting thrombolytic infusion catheter (EKOS Corporation, Bothell, WA) are available [Figure 1]. However, due to its high-cost, they both are reserved only for those patients who do not respond well with PMT.
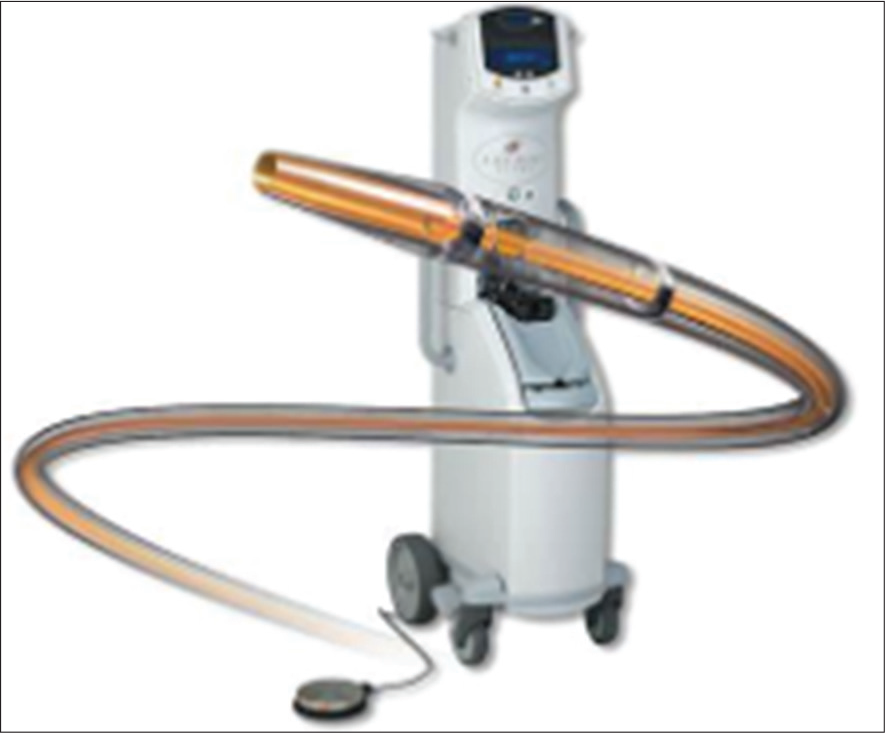
- AngioJet catheter system.
Percutaneous transluminal angioplasty and stenting
After successful PMT, the patency of proximal venous pathway should be demonstrated by angiography. If significant residual stenosis is present, serial dilatation is done with increasing balloon size, after crossing the lesion with wire, up to the size appropriate to the size of the vein. After this, self-expanding stent of sufficient size and length may be deployed, most commonly, the wall stent. Post-dilatation may be required to overcome the stent under expansion due to venous fibrosis, common in chronic venous occlusion. In follow-up, technical success of procedure is demonstrated by DSA which should have residual stenosis of <30%.[9,10]
Inferior vena cava (IVC) occlusion
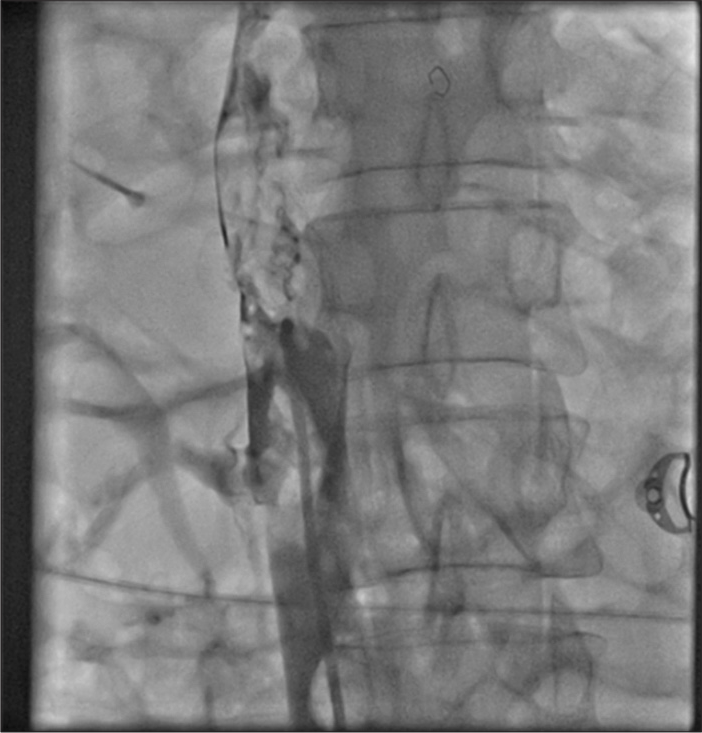
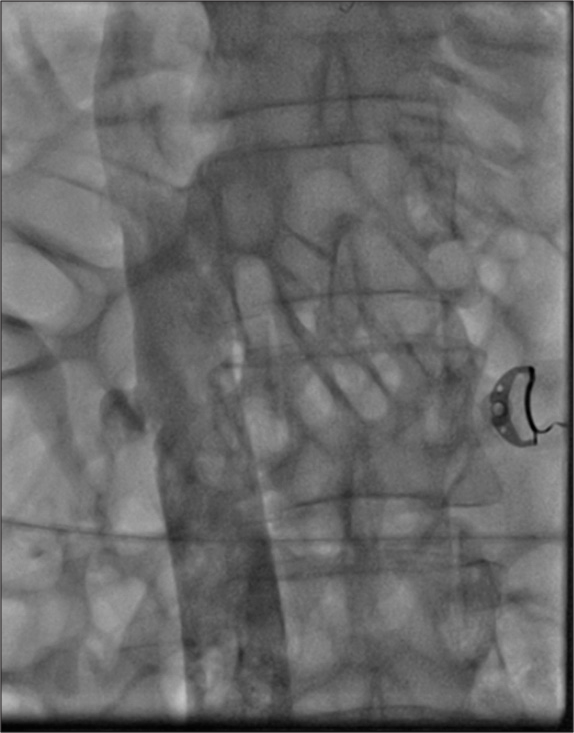
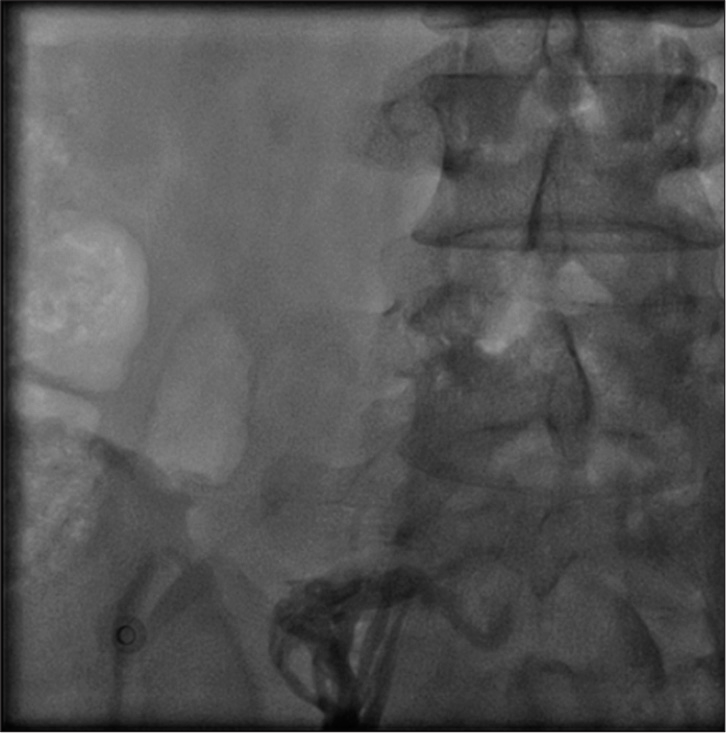
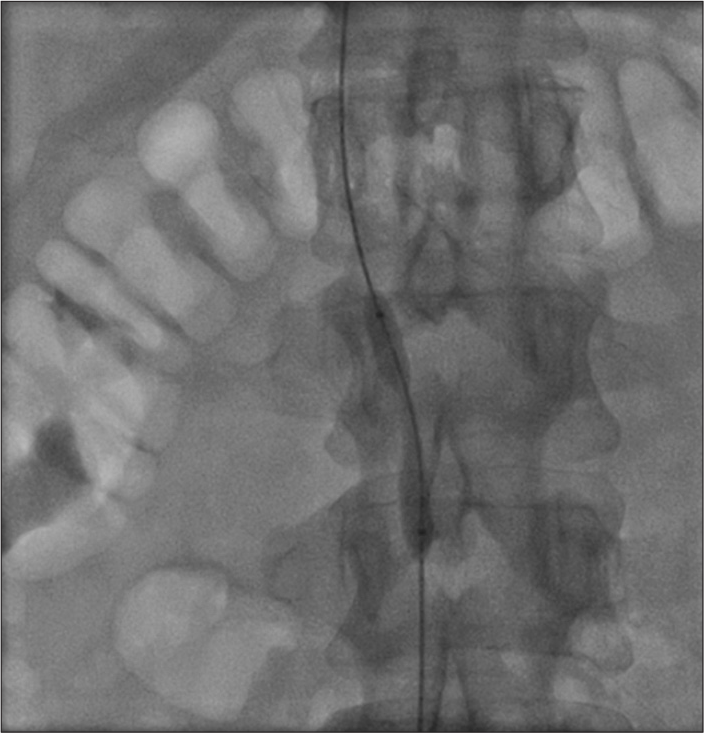
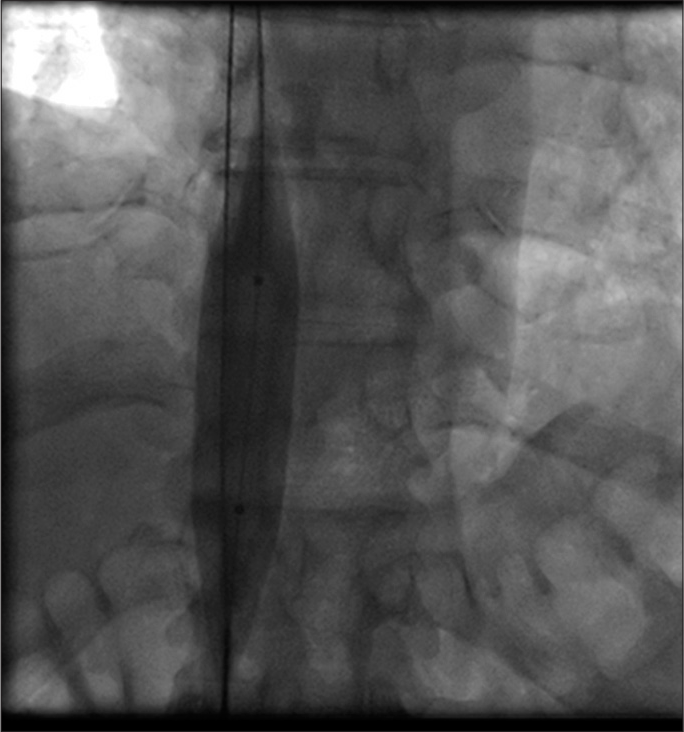
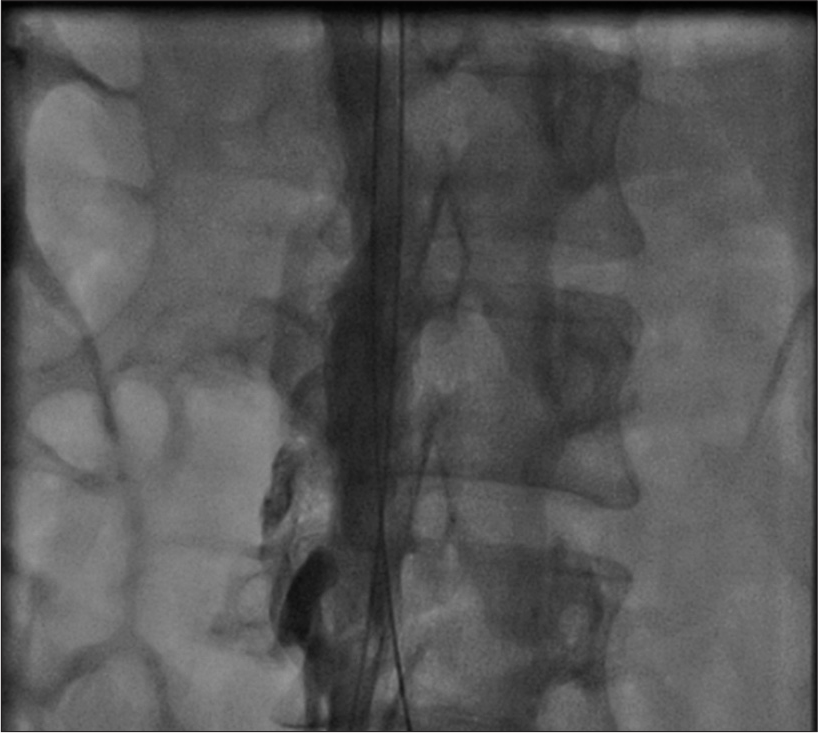
Thrombotic occlusion of IVC mostly occurs due to propagation of iliofemoral vein thrombosis or IVC filter thrombosis [Figure 2]. Most of the patients present with unilateral or bilateral lower limb swelling. However, some patients have good collateral pathways and so may not manifest classical symptoms of IVC obstruction. Standard treatment for IVC thrombosis involves surgical thromboembolectomy.[11,12] Initial endovascular treatment consists of CDT [Figure 3], for acute occlusion followed by PMT [Figures 4 and 5]. Usually, large sheaths are required, through femoral vein approach. Underlying stenosis requires angioplasty with or without stent placement. Self-expanding stents are used which can be oversized to prevent stent migration. If IVC obstruction is chronic [Figure 6], the role of CDT is limited. The occlusion is difficult to cross. Mostly, terumo wire is used to cross and the serial dilatations are given with increasing sized balloons. These lesions are very fibrotic and require high pressures to dilate [Figure 7], which may cause significant pain to the patient.[13] Sizes most often used are up to 14 mm for iliacs and may be up to 24 mm for IVC [Figures 8 and 9].
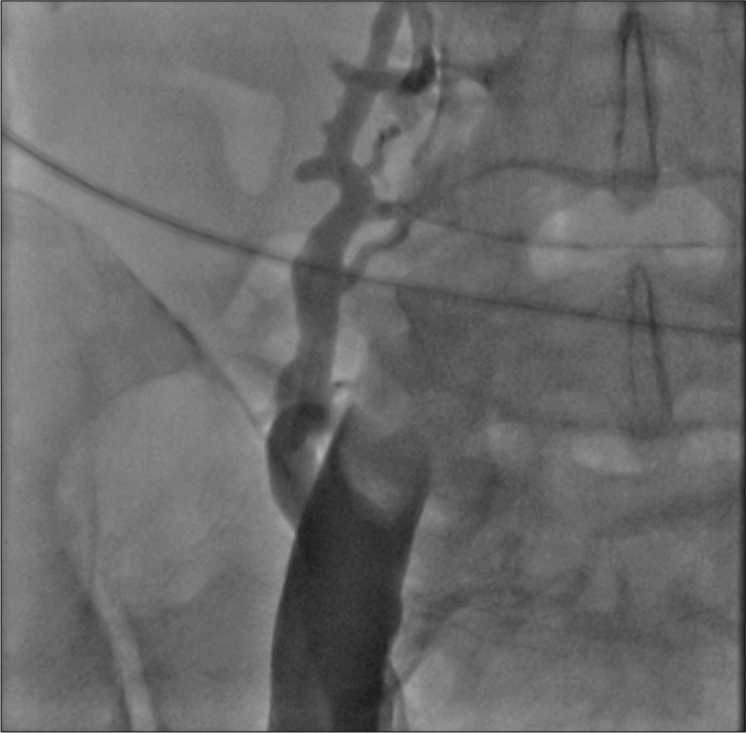
- Acute occlusion of inferior vena cava, with thrombus.
- Partial recanalization of inferior vena cava after catheter directed thrombolysis.
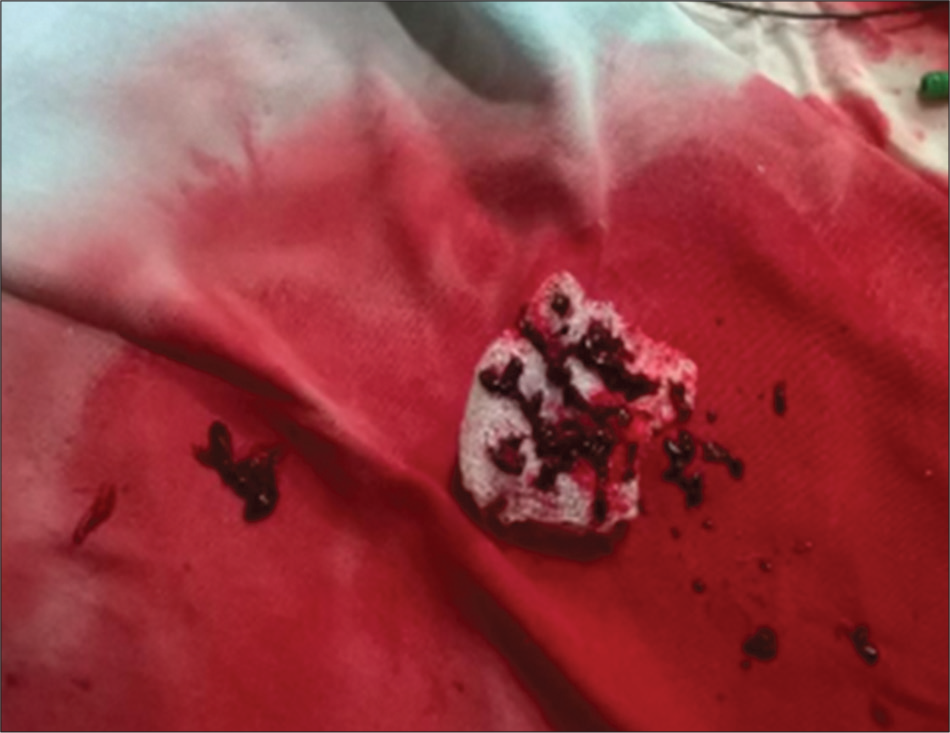
- Thrombus extracted manually.
- Complete recanalization of inferior vena cava after catheter directed thrombolysis and mechanical thrombus extraction.
- Chronic occlusion of inferior vena cava.
- Fibrotic lesion of inferior vena cava, difficult to dilate with 7 mm balloon.
- Dilatation of inferior vena cava with 18 mm balloon.
- Recanalization of inferior vena cava after balloon dilatation.
Endovascular management of May–Thurner syndrome (MTS)
MTS is defined as compression of the left iliac vein by the right common iliac artery against lumber spine, resulting in thrombosis of iliac vein or iliofemoral vein. Risk factors include female gender, especially, postpartum period, multiparous women, or who are using oral contraceptives. Scoliosis can also predispose to MTS. Other risk factors include dehydration, hypercoagulable states, and radiation exposure. Among patients who present with a symptomatic lower extremity venous disorder, MTS is estimated to be the etiology in 2–5% of patients, although some retrospective reviews have reported much higher rates.[14] Majority of individuals who are having a MTS anatomy are usually asymptomatic, but can have progression to venous thrombosis due to venous hypertension. Patients who are symptomatic due to MTS anatomy can have swelling with severe debilitating acute extremity pain, venous claudication, or chronic symptoms of venous insufficiency. A significant number of patients (up to 85%) with MTS experience venous claudication.[15,16] This phenomenon is due to venous outflow obstruction.
Non-invasive venous imaging
In the absence of thrombosis, in MTS, non-invasive vascular imaging can be done to identify the lesion. Options include duplex ultrasound, CT venography, and MR venography.
Although venous ultrasound has high sensitivity and specificity for the detection of proximal DVT using B-mode using compressibility criterion, the deep location of the proximal iliac vein along with other factors (e.g., obesity) may limit ultrasound for making an accurate diagnosis of MTS. B-mode will help to compare the reduction in the diameter of the vein at the smallest area to that of normal diameter of the vein. Peak vein velocity is the measurement of post-stenotic segment to that of the pre-stenotic segment and gradient >2.0 is considered significant.[17]
Both CT and MR venograms have more than 95% specificity and sensitivity for diagnosing MTS, but these imaging modalities require adequate technical protocols for imaging acquisition.[18,19]
The treatment of this syndrome (MTS) depends on whether DVT is present or not. The treatment is usually conservative in patients with non-thrombotic MTS with no or mild symptoms, whereas in patients with non-thrombotic MTS with moderate to severe symptoms (leg swelling, skin discoloration and pain), the treatment is usually angioplasty and stenting. In thrombotic MTS, initial treatment consists of anticoagulants, CDT or PMT. Then, USG is done to assess venous stenosis; and if found, angioplasty and stenting of iliocaval segment are done. Extending the stent into the IVC has no negative impact.[20]
Axillosubclavian vein thrombosis (ASCT)
Upper limb DVT is rare and accounts for 2–4% of all DVT.[21] It is mostly secondary to indwelling catheters or pacemaker leads. Most patients can be managed anticoagulants of short duration and may or may not require catheter removal. In presence of severe symptoms, CDT may be required.
Primary ASVT (Paget-Schroetter Syndrome) is less common than secondary ASVT. It affects people of younger age group which is healthy otherwise. Repetitive activity of the upper extremity predisposes susceptible individuals to ASCT in the presence of an underlying abnormality of the thoracic outlet. The subclavian vein is usually compressed between the first rib and a hypertrophied scalene muscle and subclavius tendon. Medical therapy usually fails in this subgroup of patients. An effective treatment is resection of this first rib. Initial therapy is Catheter-based treatment, which begins with basilic vein access, followed by CDT. Then, the early first rib resection is performed in the same hospital sitting.
Superior vena cava (SVC) syndrome
SVC syndrome occurs as a result of obstruction of flow through SVC. There are multiple causes of SVC syndrome [Table 2], but most common cause is intra-thoracic malignancy, responsible for 60–85% cases.[22] Signs of SVC syndrome include swelling of face, neck, and upper limb with distension of jugular vein. Symptoms include breathlessness, chest discomfort, cough, difficulty in swallowing, hoarseness of voice, and fullness of head due to facial plethora. Severe headache, delirium, and coma suggest the presence of associated cerebral edema. Anticoagulation is given in these conditions to prevent formation of thrombus and subsequent propagation and to reduce the risk of pulmonary embolism (PE). Revascularization is done only in the presence of significant symptoms. In SVC syndrome, palliative therapy is advocated if life expectancy is <6 months. SVC stenting for malignant disease carries an excellent technical success rate of 95–100% and primary and secondary patency rates of 85% and 93%, respectively, at 3 months.[23] Before planning endovascular intervention, bilateral upper limb venography is required to see the extent of disease. CDT is usually required followed by angioplasty with large balloons. Stents are required in most cases as the lesions are mostly fibrotic. Stents are oversized by 10–20% to prevent stent migration. If there are pacing leads, which cannot be extracted, stenting across the leads can be done and is safe.[24]
| Malignancy |
| Bronchogenic carcinoma |
| Non-Hodgkin lymphoma |
| Indwelling central venous catheters |
| Pacemakers/implantablecardiac defibrillators |
| Granulomatous infection (e.g., tuberculosis) |
| Mediastinal fibrosis and sclerosing mediastinal fibrosis |
| Histoplasmosis |
| Post-radiation |
BUDD–CHIARI SYNDROME (BCS)
BCS is an uncommon disorder characterized by obstruction of hepatic venous outflow provided that obstruction is not due to cardiac disease/pericardial disease, or due to the obstruction of sinusoids (veno-occlusive disease). Primary BCS is defined as when there is a predominantly venous obstruction (thrombosis or phlebitis), whereas secondary BCS is defined as a condition when there is compression or invasion of the hepatic veins and/or the IVC by a lesion that originates outside of the vein (e.g., a malignancy). Symptomatic BCS has a high mortality rate if untreated. In a study, performed before specific therapy became available, 90% patients died within 3 years.[25] With treatment, survival rates are good. In a series of 163 patients who were treated, survival rates were 87, 82, and 74% at 1, 2, and 5 years, respectively.[26]
Initial treatment consists of correcting the predisposing condition. Injectable anticoagulants are started, and finally, treatment of complications of portal hypertension is done. Thrombolysis is done in acute BCS, if there is well defined clot on venography. Percutaneous angioplasty and stenting can be done in patients of acute or subacute BCS who are symptomatic, which provided that this obstruction can be subjected to angioplasty and stenting (as visualized either on MR venography or venography). Focal narrowing in hepatic vein and web in IVC can be dilated. Since recurrence rate of angioplasty is high, self-expanding stents are implanted.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
TIPS placement can be considered for patients of chronic BCS and complications of portal hypertension. Alternative treatments, if TIPS placement is not feasible or is unsuccessful, include surgical shunts and liver transplantation. In one of the largest series (which included patients with severe BCS who did not respond to medical therapy or attempts at achieving recanalization), overall 5-year survival was 84% after TIPS placement which was similar to 5-year survival from published reports of orthotropic liver transplantation for BCS.[27] At 1 and 5 years, transplant-free survival rates after TIPS placement were 88% and 78%, respectively.
ENDOVASCULAR TREATMENT OF VARICOSE VEINS (VVS)
VVs are swollen, tortuous or elongated veins. This can be due to decreased vein elasticity (primary venous reflux) or existing (inherited) valve dysfunction or damage of the valve from prior thrombotic events (secondary venous reflux). This results in venous pooling and increased pressure in the veins. This leads to ballooning out of branch vessels leading to varicosities. Symptoms include pain, aching, edema of lower limb, restless legs, and chronic venous insufficiency, and finally, venous ulcers may form. There is a strong familial predisposition to develop VVs with the risk 90% in offspring if both parents affected, 20% when neither is affected, and 45% (25% boys and 62% girls) if one parent is affected.[28] The annual incidence of varicosities is 2.6% among women and 1.9% among men and did not vary within the age range (40–89 years) studied. Surgery is the mainstay treatment for superficial veins such as the great saphenous vein (GSV) and the small saphenous vein (SSV), which are the major cause of leg VV. Endovascular techniques such as endovascular laser therapy (ELT) or radiofrequency (RF) are major treatment alternatives to surgery for varicosities. Both these techniques involve venous wall ablation through thermochemical reactions. In a recent study from UK, treatment strategy was based on USG findings and patients were then categorized with, RF for GSV diameters 3–12 mm (RF), ELT for diameters >3 mm, and foam sclerotherapy for diameters <1 mm. Out of 328 patients, 73% of the cases were amenable for at least one of the three endovascular approaches.[29]
FOAM SCLEROTHERAPY
Sclerotherapy involves scarring and closure of blood vessel by injecting a chemical agent (sclerosant). In foam sclerotherapy, air is mixed with the liquid sclerosant to create foam. When this is injected into the VV (under ultrasound guidance), it displaces the blood within the vein and fills the vein. This causes the vein to spasm and scar. The vein can be checked with the ultrasound to see if the injection has been successful. Foam sclerotherapy has a good success rate, with 80–90% of veins remaining closed after 3 years.
ELT
In this technique [Figure 10], USG is done to size the veins and to see all areas of venous reflux. Puncture of target vein is done and sheath is inserted. Through which, optical fiber carrying laser energy is introduced. A tumescent anesthetic solution is injected into the soft-tissue surrounding the target vein along its entire length. A tumescent anesthetic solution is injected around the vein. An example of a tumescent anesthetic would be a combination of 0.5 mg adrenaline or epinephrine, 4.2 mg bicarbonate, and 35 mL lidocaine diluted in 500 mL 0.9% saline. In addition to providing anesthesia, this has a compression effect on the vein, which maximizes the laser’s effect on the vein wall. Once proper position is confirmed, laser energy and laser fiber and sheath are slowly pulled back along the whole length of vein. At the end of procedure, homeostasis is achieved by applying pressure to the entry point.[30]
After the procedure, compression stockings and bandages are applied to reduce post-operative bruising, tenderness, and the risk of venous thromboembolism.

- Endovascular laser console and catheter.
RADIOFREQUENCY ABLATION (RFA)
Symptomatic superficial insufficiency of veins >3 mm in diameter which is refractory to compression stockings therapy in individuals >18 years of age is an indication for treatment. Usually, this is limited to the GSV and SSV. Access to the refluxing superficial vein is obtained with a 16 or 18 G needle under ultrasound guidance at the lowest point of its incompetence, usually around 15 cm distal to knee joint. The RF ablation catheter is then advanced under ultrasound guidance and placed at least 2 cm distal to the saphenofemoral junction. The RF generator is then activated [Figure 11], which results in segmental heat energy of 120°C being applied. The RF generator is activated in 20-s intervals until the entire length of the vein is treated. At the end of the procedure, hemostasis is achieved by manual compression at the site of venous access and catheter entry. After RF therapy of VVs, compression stockings are recommended for continued regular use. Overall, the rate of adverse effects has been reported to be as low as 4.4% to as high as 40%. Pain is the most common adverse effect that contributes to >95% of the higher reported rates.[31]
IVC FILTER IMPLANTATION
Main indications are (1) contraindications for anticoagulants use or bleeding during anticoagulation use for DVT and (2) recurrent PE in spite of adequate anticoagulant use. Filter can be placed from femoral vein route or from internal jugular route [Figure 12]. Filter may be permanent or temporary, depending on the temporary or permanent contraindication for anticoagulants. These may be placed before CDT/MT in case of large thrombus load in IVC.

- Endovascular radiofrequency console and catheter.
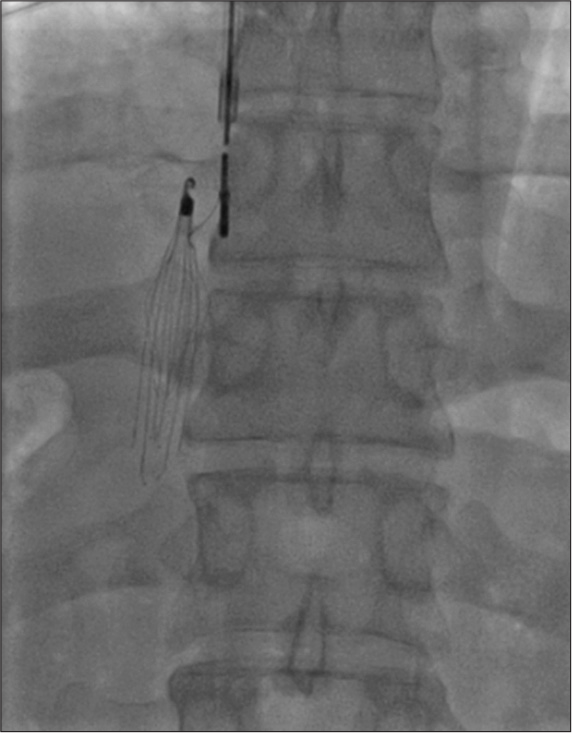
- Inferior vena cava filter placement form internal jugular approach.
CONCLUSION
Venous interventions have, now, established a definite role in treatment of various venous diseases such as upper and lower limb DVT, SVC and IVC obstruction, Budd–Chiari, MTS, and, lately, a surgical alternative to VVs. As compared to arterial interventions, which are being done for long time, venous interventions require a longer learning curve and should be started under expert guidance.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Early thrombus removal strategies for acute deep venous thrombosis: Clinical practice guidelines of the society for vascular surgery and the American venous forum. J Vasc Surg. 2012;55:1449-62.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11:CD011536.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention and treatment of the postthrombotic syndrome. J Vasc Surg. 2010;52:21S-8S.
- [CrossRef] [PubMed] [Google Scholar]
- The success rate of fibrinolytic therapy in fresh and old thrombosis of the iliac and femoral veins. Angiology. 1983;34:61-9.
- [CrossRef] [PubMed] [Google Scholar]
- Catheter-directed thrombolysis for lower extremity deep venous thrombosis: Report of a national multicenter registry. Radiology. 1999;211:39-49.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): A randomised controlled trial. Lancet. 2012;379:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377:2240-52.
- [CrossRef] [PubMed] [Google Scholar]
- Quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014;25:1317-25.
- [CrossRef] [PubMed] [Google Scholar]
- Iliofemoral deep venous thrombosis: Aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487-94.
- [CrossRef] [PubMed] [Google Scholar]
- Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006;17:417-34.
- [CrossRef] [Google Scholar]
- Obstructive lesions of the inferior vena cava: Clinical features and endovenous treatment. J Vasc Surg. 2006;44:820-7.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical removal of an inferior vena cava thrombus. Eur J Vasc Surg. 1992;6:78-82.
- [CrossRef] [PubMed] [Google Scholar]
- A dangerous miss-angioplasty of chronic total occlusion of entire length of inferior vena cava and bilateral iliac veins. J Invasive Cardiol. 2019;31:E155-6.
- [Google Scholar]
- May-thurner syndrome and other obstructive iliac vein lesions: Meaning, myth, and mystery. Vasc Med. 2015;20:74-83.
- [CrossRef] [PubMed] [Google Scholar]
- Venous claudication in iliofemoral thrombosis: Long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118-26.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320. discussion 327-8
- [CrossRef] [PubMed] [Google Scholar]
- Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg. 2007;46:101-7.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance venography in the diagnosis and management of may-thurner syndrome. Vasc Endovascular Surg. 2002;36:51-7.
- [CrossRef] [PubMed] [Google Scholar]
- Computed tomography findings in 10 cases of iliac vein compression (may-thurner) syndrome. Eur J Radiol. 2005;55:421-5.
- [CrossRef] [PubMed] [Google Scholar]
- Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979-90.
- [CrossRef] [PubMed] [Google Scholar]
- Upper-extremity deep vein thrombosis: A prospective registry of 592 patients. Circulation. 2004;110:1605-11.
- [CrossRef] [PubMed] [Google Scholar]
- The superior vena cava syndrome: Clinical characteristics and evolving etiology. Medicine (Baltimore). 2006;85:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Follow-up results of 71 patients undergoing metallic stent placement for the treatment of a malignant obstruction of the superior vena cava. Cardiovasc Intervent Radiol. 2007;30:959-67.
- [CrossRef] [PubMed] [Google Scholar]
- Placement of SVC stents over pacemaker wires for the treatment of SVC syndrome. J Vasc Interv Radiol. 2000;11:215-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Budd-Chiari syndrome: Correlation between hepatic scintigraphy and the clinical, radiological, and pathological findings in nineteen cases of hepatic venous outflow obstruction. Gastroenterology. 1975;68:509-18.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167-75.
- [CrossRef] [PubMed] [Google Scholar]
- TIPS for Budd-Chiari syndrome: Long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135:808-15.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of the familial factor in varicose disease. Clinical study of 134 families. J Dermatol Surg Oncol. 1994;20:318-26.
- [CrossRef] [PubMed] [Google Scholar]
- Suitability of varicose veins for endovenous treatments. Cardiovasc Intervent Radiol. 2009;32:988-91.
- [CrossRef] [PubMed] [Google Scholar]
- Endovenous laser treatment for uncomplicated varicose veins. Phlebology. 2009;24(Suppl 1):50-61.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized clinical trial of endovenous laser ablation versus direct and indirect radiofrequency ablation for the treatment of great saphenous varicose veins. Br J Surg. 2019;106:998-1004.
- [CrossRef] [PubMed] [Google Scholar]
- Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997;8:405-18.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacomechanicalthrombec-tomy for iliofemoral deep vein thrombosis: An alternative in patients with contraindications to thrombolysis. J Vasc Surg. 2009;50:1092-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results using catheter-directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:112-7.
- [CrossRef] [PubMed] [Google Scholar]







