Translate this page into:
Transcatheter Aortic Valve Replacement – “Beyond the Procedure”
*Corresponding author: Veena Nanjappa, Department of Cardiology, Sri Jayadeva Institute of Cardiovascular Science and Research Centre, Bengaluru, Karnataka, India. veenananjappa@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Nanjappa V, Batra T. Transcatheter Aortic Valve Replacement – “Beyond the Procedure.” Indian J Cardiovasc Dis Women. 2025;10:67-77. doi: 10.25259/IJCDW_67_2024
Abstract
Transcatheter aortic valve replacement (TAVR) is a rapidly proliferating technology with the potential to become the dominant treatment strategy for aortic valve stenosis in patients not only for excessive or high operative risks but also intermediate risk patients. A systematic description of transcatheter heart valve failure has not been analyzed. In the coming days, we shall be seeing more and more TAVR patients who shall have unique challenges in their post-procedural care. And many a times, the procedure itself would have been the easiest part of their TAVR journey. Post TAVR care needs to be a continuous and concerted team effort involving patient and care giver.
Keywords
Transcatheter aortic valve replacement failure
Transcatheter aortic valve replacement infective endocarditis
Transcatheter aortic valve replacement late stroke
Transcatheter aortic valve replacement prosthetic valve thrombosis
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) is increasingly being embraced in both developed and developing nations as a treatment modality for severe aortic stenosis. It has the potential to become the primary treatment option not only for patients at high surgical risk but also for those classified as intermediate risk. Market research has estimated the TAVR market in 2020 which was 4559 million dollars to a fourfold increase to 16,937 million dollars in 2030. Asia pacific region is expected to register the highest increase.
It is important to study the valve durability, mechanisms of transcatheter heart valve (THV) failure and long-term efficacy. Approximately, 200,000 patients worldwide undergo surgical aortic valve replacement. The modes of failure of surgical bio prostheses, including infective endocarditis (IE), thrombosis, and structural valve failure (SVF), have been clearly described. In contrast, comprehensive analysis and evaluation of THV failure, as outlined in Table 1, is yet to be done. Knowledge gap exists in the current understanding of THV performance and outcomes. In the coming days, we shall be seeing more and more TAVR patients who shall have unique challenges in their post procedural care. And many a times, the procedure itself would have been the easiest part of their TAVR journey. Post TAVR care needs to be a continuous and concerted team effort involving patient and caregiver.
| Valve prosthesis failure | THV specific failure | Others |
|---|---|---|
| Infective endocarditis | Paravalvular leak aortic regurgitation | Coronary disease |
| Prosthetic valve thrombosis | Prosthesis compression | Conduction abnormalities |
| Structural failure | Late prosthetic embolization | Late stroke |
THV: Transcatheter heart valve
IE OF TAVR VALVE
The incidence of TAVR IE is about 1.1% as per the data from the multicenter registry from Italy and Germany.[1] Incidence of surgical prosthetic valve IE is 03–1.2%/patient year. There was no difference in rates of surgical and TAVR endocarditis in the placement of aortic transcatheter valves (PARTNER) trial. The median time of manifestation of IE is in the first 6 months [Table 2].
| Onset of IE post TAVR | Percentage |
|---|---|
| <60 days | 18% |
| 60 days–1 year | 62% |
| >1 year | 20% |
IE: Infective endocarditis, TAVR: Transcatheter aortic valve replacement
Clinical risk factors for IE
Diabetes,
Chronic kidney disease,
Immunosuppression,
Poor oral hygiene,
and recurrent infections.
Procedural risk factors
Severe paravalvular aortic regurgitation (PAR)
Redo procedures (including TAVR in a prior TAVR)
A low TAVR implantation that interferes with mitral valve closure and generates turbulences; 24% of TAVR endocarditis cases had documented “satellite” endocarditis of the mitral valve due to direct contact with the mitral apparatus
High transvalvular gradients (>50 mmHg) as well as
Vascular access-site complications.
Blood culture tests may be positive in 79% cases in a study.[2] Patients present mostly with fever and heart failure. Coagulase-negative Staphylococci (CONS) and Streptococci were the predominant causative microorganisms. Enterococci species is seen in 25–30% cases.
Echocardiographic findings (positivity 86%) may include: Mobile vegetations, progressive stenosis, or regurgitation abscess formation.
Conduction disturbances can also occur due to its anatomical proximity.
There were no changes in the rates of IE with the choice of access whether it was transfemoral or transapical vascular access.
Mylotte et al.[2] reported a survival of 75% whereas Latib et al.[1] reported in-hospital survival of only 38%. In a registry data[3] of 250 cases, in-hospital mortality rate was found to be 36%. Surgery is recommended as per guidelines.[4] Those with complicated IE cases (those patients with congestive cardiac failure, large vegetations, and paravalvular disease), it may not always be feasible to subject them for surgery as they are prohibitively at high surgical risk.
Antimicrobial prophylaxis
Administered 0–120 min, preferably an hour before arterial puncture with:
Single dose and of intravenous (iv) amoxicillin/clavulanic acid – 2.2 g
iv Cefazolin 2 g or cefuroxime 1.5 g
Single dose of intravenous Vancomycin 15 mg/kg or intravenous Teicoplanin 9–12 mg/kg in patients with beta-lactam allergy or in the presence of high prevalence of methicillin resistant Staphylococcus aureus (MRSA)
Table 3 outlines some of the steps to reduce IE.
| Cardiac catheterization laboratory | Patient preparation | Operators and staff |
|---|---|---|
| Adequate Fumigation | Shower with chlorhexidine soap | Surgical hand hygiene, mask, and hood |
| Minimal personnel inside catheterization laboratory | Decolonization with nasal mupirocin for 5 days may be considered for nasal carriers of Staphylococcus aureus especially if they are obese and diabetic. | Change gloves or remove outer glove before having contact with the unpacked valve. |
| Closed doors | Access site hair removal with clippers | |
| Minimizing exposure time of unpacked THV prosthesis to ambient air to <15 min is advised. | Peri-procedural skin disinfection with three applications of alcohol-based disinfectant | |
| Antibiotic prophylaxis |
THV: Transcatheter heart valve
Antibiotic prophylaxis before other procedures
Any dental procedures that involve manipulation of gingival tissue, manipulation of the periapical region of teeth, or perforation of the oral mucosa requires prophylaxis against THV endocarditis. It is not necessary requisite before transesophageal echocardiography, esophageal gastroduodenoscopy, colonoscopy, or cystoscopy in the absence of active infection.
PROSTHETIC VALVE THROMBOSIS (PVT)
THV thrombosis is rare. PARTNER trial did not report any cases of clinically significant prosthesis thrombosis nor did large TAVR registries.[5,6]
A thrombus is classified as PVT as per the VARC criteria[7] if it is attached to or near an implanted valve; if it occludes the blood flow; if it interferes with the valve function, or if it is sufficiently large to warrant treatment.
PVT of a TAVR prosthesis is very rare (up to 0.8%). It usually occurs at a mean time of 9 ± 7 months after the THV implant.[2]
Risk factors
Coexisting prothrombotic conditions,
Incomplete expansion and/or apposition to the aortic wall, and
Native leaflets overhanging the balloon-expandable systems.
Symptoms consist mainly of a progressive dyspnea. Manifest PVT was associated with stroke, cardiogenic shock, and death.[8]
-
Echocardiographic findings include:
Increased transvalvular gradients,
Leaflet thickening and
Direct visualization of thrombus.
Stable patients can be treated with oral anticoagulation which has shown to decrease transvalvular gradients and restore leaflet mobility. There were no reported cases of PVT in PARTNER randomized trials or in large TAVI registries.[9,10] Obstructive PVT should be considered for surgery or valve in valve (VIV) procedures with cerebral protection. Very few reports are available of use of thrombolysis in obstructive PVT of THV.
Subclinical leaflet thrombosis
-
On four-dimensional volume-rendered computed tomography (4DCT), it appears as hypo-attenuating leaflet thickening (HALT). HALT can limit the movement of the leaflets and is referred to as hypo-attenuation affecting motion (HAM).[3] This phenomenon can have significant implications for the functionality of the valve. A timeline course of case is given in Figure 1a-f.
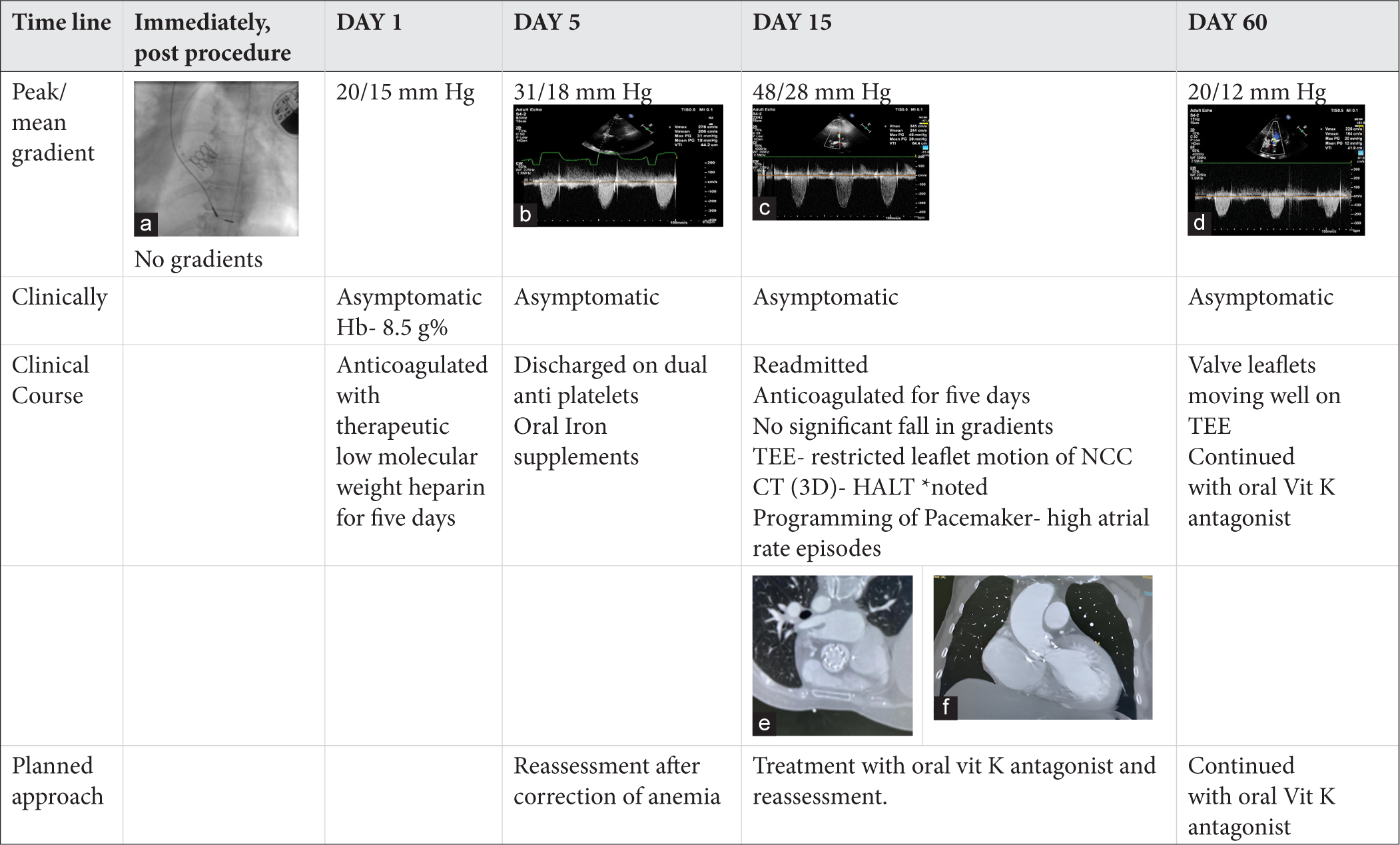 Figure 1:
Figure 1:- Time line course of a case of a 69 year old lady who underwent TAVR with 20 mm balloon expandable valve for severe symptomatic Rheumatic aortic valve stenosis. She had subclinical valve motion restriction (documented on Transesophageal echocardiography) and HALT on cardiac CT with increase in transvalvular gradients which reduced after starting the patient on therapeutic oral anticoagulation with Vitamin K antagonists. (a) Deployed balloon expandable valve on fluoroscopy, (b) Peak/mean gradient across aortic valve on echocardiogram on Day 5 of the procedure, (c) Peak/mean gradient across aortic valve on echocardiogram on Day15 of the procedure, (d) Peak/mean gradient across aortic valve on echocardiogram two months after the procedure, (e and f) Demonstrating HALT on CT. * HALT- Hypoattenuated leaflet thickening. (*HALT- Hypo attenuated leaflet thickening. CT: Computer tomography, TEE: Trans Oesophageal echocardiography, NCC: Non coronary cusp).
-
Risk factors for subclinical leaflet thrombosis:
Under-expansion of the stent frame
Post-dilatation of self-expanding THV reduces the risk.
In a recent 4DCT sub-study of the PARTNER 3 trial, it was observed that 50% of the leaflets exhibited HALT at the 30-day mark following TAVR were without thrombosis 1- year post-procedure even without oral anticoagulation, whereas HALT appeared in 20% of patients at 1 year despite normal leaflet at 30 days.[8]
A 5-year follow-up study[11] indicated that around 20% (27 out of 124) of patients who underwent TAVR developed HALT within the 1st year. However, this occurrence did not significantly impact the risk of subsequent adverse cardiovascular events or the performance of the valve over a 5-year period, even in the absence of additional anticoagulation therapy.
A 4D CT trial comparing apixaban with antiplatelet showed that HALT was reduced in patients on apixaban but in patients who had indication for anticoagulation, it was not superior than Vitamin K antagonist (VKA).[12]
POST TAVR ANTITHROMBOTIC THERAPY
Single-antiplatelet therapy is preferred in post-TAVR patients who have no indications for oral anticoagulation (Class 2a, Level of Evidence [LOE]: B). Pre-procedural loading dose of aspirin has not been proven useful. Dual-antiplatelet therapy with aspirin (75–100 mg) and clopidogrel 75 mg is a class IIb recommendation.[8] Anti-coagulation with a VKA for at least 3 months (Class 2b, LOE: B) may be considered in patients at low risk for bleeding. Patients on oral anticoagulation will require careful prothrombin time international normalised ratio (PT INR) monitoring. There are notable gaps in the evidence regarding the use of direct oral anticoagulants in patients with atrial fibrillation (AF) with TAVR [Figure 2].
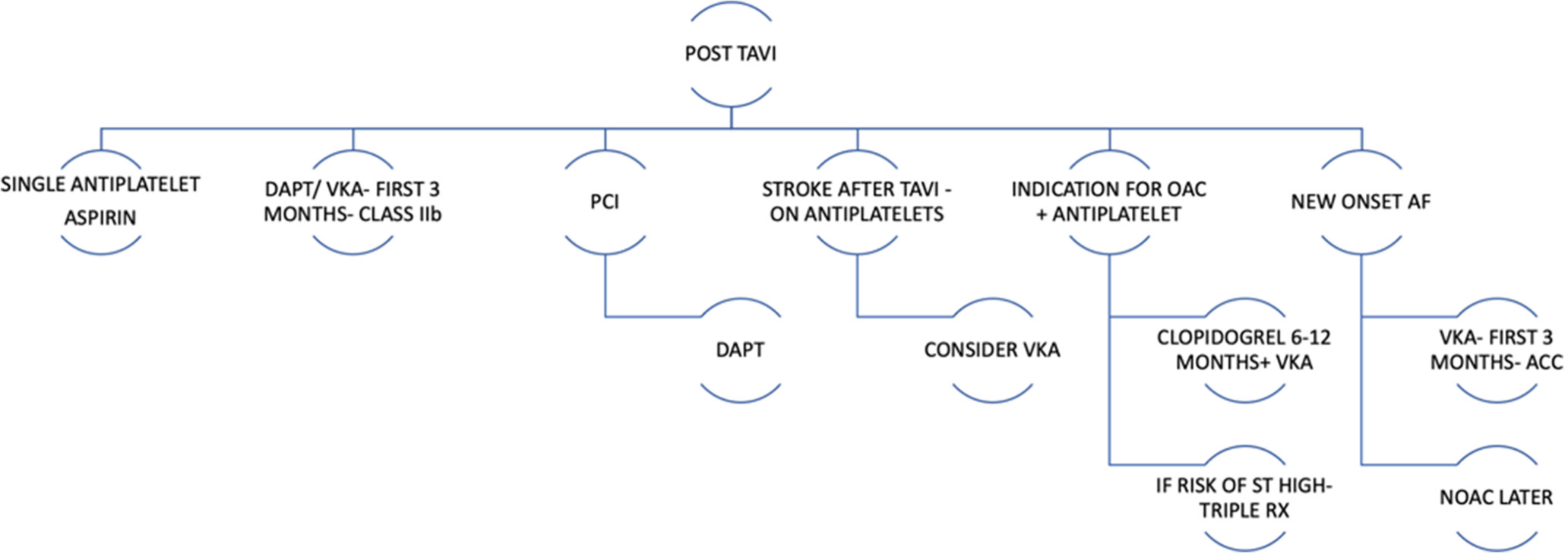
- Antiplatelet therapy post TAVR. (PCI: Percutaneous coronary intervention, DAPT: Dual antiplatelet therapy, VKA: Vitamin K antagonists, OAC: Oral anticoagulation, AF: Atrial fibrillation, NOAC: Novel oral anticoagulants).
The efficacy of clopidogrel in elderly patients is debatable for several reasons.[13,14] They are at an increased risk of bleeding which may necessitate treatment cessation. Less than one-third patients require dual-antiplatelet therapy for patients who undergo TAVR with percutaneous coronary intervention (PCI) and stenting. Patients with THV and AF or with high CHA2DS2-VASc scores require anticoagulation rather than clopidogrel.
CORONARY ARTERY DISEASE POST TAVR
The incidence of acute coronary syndrome (ACS) following TAVR is approximately 10% after a mean follow-up period of 2 years. Vilalta et al. in their study of 774 over 25 months follow-up showed that 10% of patients had ACS in TAVR cohort[15] with majority occurring within a year post-TAVR. Non ST elevation myocardial infarction (NSTEMI) was the most common presentation. Prior history of coronary artery disease or revascularization, presence of diabetes, acute kidney injury, and VIV[16] were predictors of ACS. ACS post–TAVR has poor prognosis with very high in-hospital (10%) and late mortality rates. It is mainly related to difficult coronary access.[17] ST-elevation myocardial infarction (STEMI) following TAVR is associated with high mortality rate. One-third of STEMI patients do not survive beyond 30 days. In a patient with stable coronary artery disease, timing of PCI is dependent on whether the patient is symptomatic and whether the lesion is proximal with demonstrable ischemia [Figure 3].
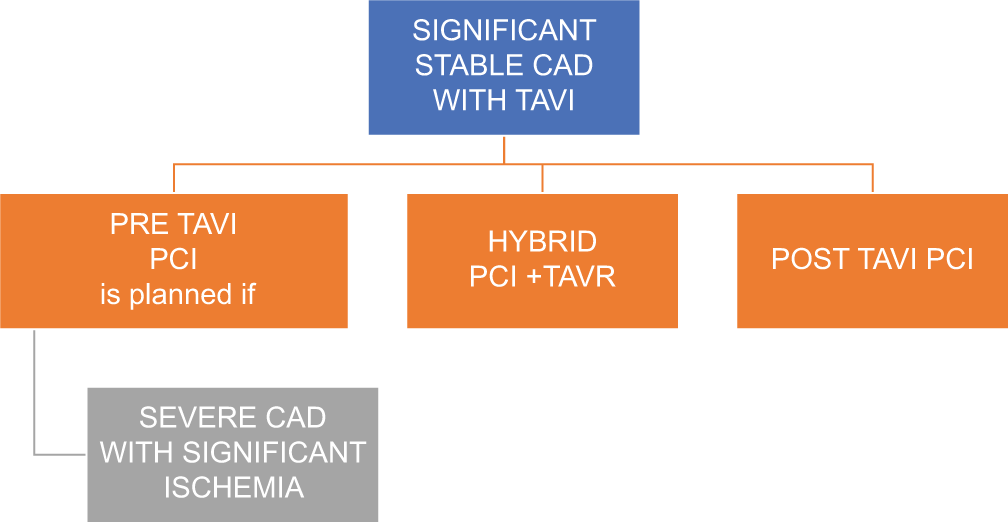
- Timing of PCI in patients with stable CAD. (PCI: Percutaneous coronary intervention, TAVR: Transcatheter aortic valve replacement, CAD: Coronary artery disease).
Challenges in coronary access in a patient post TAVR
Coronary anatomy that presents challenges during the periprocedural phase can also create difficulties for subsequent coronary access during PCIs. This subset of patients may require careful consideration and planning to navigate these anatomical complexities effectively.
Factors associated with difficult access
Commissural Misalignment – Commissural alignment is now available with most valve systems which further reduce the risk of difficult access. Cuspal overlap (right–left cusp fusion) view is the preferred as it commissurally aligns the transcatheter aortic valve. The left and right coronary arteries should originate from their respective commissures at a precise angle of 60° for optimal commissural alignment. Any deviation from this angle, whether smaller or larger, may suggest a misalignment of the coronary arteries.
The rotation angle needs to be determined which allows alignment of nadir of THV leaflets with the coronary ostia. This should maintain a 60° angle between the coronary ostia and the THV commissural post.
Low coronary heights.
Elongated calcific leaflets.
Small height of open frame cells.
Shorter frame as seen in balloon expandable valves (BEVs) poses less difficulty for future coronary access. Newer generation self-expanding valves have mitigated this challenge by introducing large open cell designs.
Avoiding high implants in patients with low coronary heights especially with self-expanding valve systems needs to be borne in mind.
Valve to commissure distance <4 mm especially in VIV case.
Externally mounted leaflets (as in trifecta valve) for VIV cases
Sinus sequestration: (Especially in VIV) The upward movement of the leaflets of the failed aortic THV can lead to formation of a “neoskirt,” which can cause sinus sequestration, especially if the length of the displaced leaflets is greater than the height of the sinotubular junction (STJ). Such a scenario may render coronary re-access difficult or impossible. To evaluate the risk of sinus sequestration, the valve-to-sinotubular junction (VT-STJ) distance can be measured using multidetector computed tomography. A VT-STJ measurement of <2.5– 3.5 mm is considered to indicate a high risk for sinus sequestration which may make coronary access difficult.
Nuances of coronary access
A baseline root aortogram may help delineate the coronary anatomy in relation to the THV. Left anterior oblique projection is used for delineating the origins of left main coronary artery (LMCA) and right coronary artery (RCA). Standard catheters can be used to engage BEVs. A case example is given in Figure 4.
![A 70 year old lady had new onset regional wall motion abnormalities on echocardiogram a year following TAVR with balloon expandable valve with class II effort angina. Panel a] non selective left sinus angiogram. Panel b and c] guide catheter engagement and near selective angiogram following wiring of LCX. TAVR: Transcatheter aortic valve replacement, LCX: Left circumflex artery.](/content/148/2025/10/1/img/IJCDW-10-067-g004.png)
- A 70 year old lady had new onset regional wall motion abnormalities on echocardiogram a year following TAVR with balloon expandable valve with class II effort angina. Panel a] non selective left sinus angiogram. Panel b and c] guide catheter engagement and near selective angiogram following wiring of LCX. TAVR: Transcatheter aortic valve replacement, LCX: Left circumflex artery.
Post-TAVR coronary angiographic study showed that coronary ostia were anatomically in unfavorable position (coronary ostium was below the upper part of the skirt or in front of the commissures of the TAVR valve) in 8% (RCA) to 15% left coronary artery (LCA) of cases with Sapien-3 valves, compared with 25% (RCA) to 35% (LCA) of cases with Evolut valves.[18]
Cannulation failure rate of 7.7% was noted mostly with Evolut valve in reobtain coronary ostia cannulation beyond transcatheter aortic valve stent (RE-ACCESS) study[19] where coronary angiography was systematically performed in post-TAVI cases. The study comprised one-third Evolut valve, one-third Sapien 3 valve, and one-third Accurate Neo valve.
The uppermost cell closest to the coronary ostia should be used to engage the coronaries in a self-expanding valve. The preferred access route is the right radial artery, followed by the right femoral artery. It is important to exchange catheters while being visually guided by a J-tipped 0.035 Teflon wire under fluoroscopy. It is important to direct the Teflon wire within the valve frame and not outside of it. Half-size smaller Judkins left catheter is preferred for engaging the left coronary artery, followed by the Judkins right 4, Ikari left 1/1.5 catheter. Extra back up catheters have to be avoided as they are at risk of entrapment in the valve frame. The Judkins right catheter, Ikari right 1.0, Multipurpose, and Amplatz right catheters are preferred for engaging the right coronary artery.
Non-selectively wiring the coronary vessel and later threading the guide extension and guiding catheter on the wire will facilitate selective angiography. Otherwise, a coronary wire can be introduced while the guide catheter is non-selectively engaged, and a short low profile balloon can be introduced on the wire. The wire and balloon shaft provide the necessary support for selectively threading the guide catheter. Second option is to balloon anchor the wire in a side branch and thread the guide catheter for selective intubation.
Role of leaflet reduction procedures
Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) is a transcatheter electro-surgical procedure to intentionally lacerate the leaflet to prevent coronary occlusion and is especially useful in VIV cases.
In this procedure, a wire loop is formed by snaring the Astato XS 20 guidewire which is advanced through the base of aortic leaflet into the left ventricular outflow tract (LVOT) by pre- placed snare in LVOT. A non-insulated, non-coated segment of the wire is placed at the aortic leaflet to facilitate its splitting, with electrical energy applied to achieve this. This technique aims to minimize coronary obstruction after valve implantation by separating either the bioprosthetic or native leaflet. Calcified and thickened leaflets are difficult to lacerate.
The Bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. (BASILICA) registry[20] is a prospective, multicenter study designed to monitor patients at risk for iatrogenic coronary occlusion following TAVR using the BASILICA technique. This registry has enrolled a total of 214 patients across North America and Europe, with 72.8% of these patients receiving bioprosthetic aortic valves. The results indicate a high success rate of 94.9% for leaflet traversal and 94.4% for leaflet laceration. In addition, the prevalence of coronary obstructions, whether partial or complete, was reported at 4.7%.
The EURO-BASILICA study[21] is the first multicenter study in Europe utilizing BASILICA technique. This 1-year clinical study included 76 patients from ten centers, with 5.3% having native aortic valves, 92.1% with surgical bioprosthetic valves, and 2.6% with transcatheter valves. It studied the effectiveness of the procedure in preventing coronary occlusion. About 11.8% of patients required BASILICA procedure for both coronary cusps. The technique achieved a success rate of 97.7%. About 2.4% patients had complete coronary occlusion.
SVF OF THV
Structural failure (SVF) of TAVR valves is very rare. The causal pathology for structural degeneration of THV is very similar to that of surgical bio-prostheses [Table 4]. Chronic mechanical stresses on the leaflet tissue, glutaraldehyde fixation, residual leaflet antigenicity, and systemic atherosclerosis may also contribute to the pathogenesis of structural valve failure.[22,23] It can present with severe aortic stenosis as a result of severe leaflet calcification or pannus formation and can also present with severe aortic regurgitation (AR) due to cuspal rupture. Multimodality imaging may be required to differentiate it from prosthetic valve thrombosis. The treatment of choice is valve replacement. Asymptomatic cases are managed conservatively; symptomatic patients are advised to undergo surgical aortic valve replacement.
 |
No patients in either the surgical or transcatheter groups had structural degeneration in the PARTNER trial (Cohort A) at 2 years of follow-up. Long-term data will be required to demonstrate the true incidence of SVF associated with TAVR. It may be difficult to accurately assess the SVF rates in elderly high-risk TAVR recipients. Valve durability is related to the age of the patient at the time of implantation and the time since implantation. Randomized study data are not available in elderly high-risk group. Aortic regurgitation is classified as valvular and paravalvular [Figure 5].
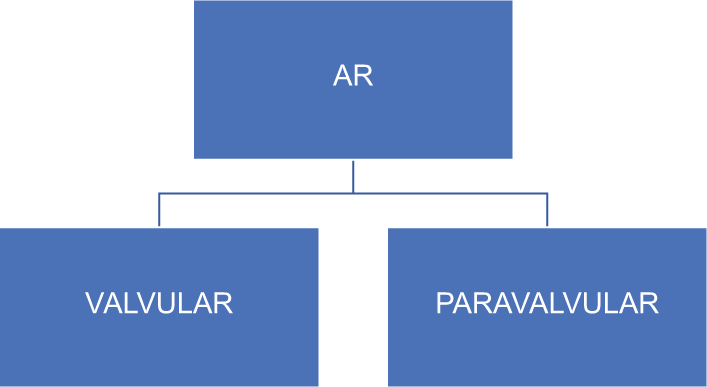
- Mechanism of AR. (AR: Aortic regurgitation).
POST-TAVR AR
The incidence of moderate-to-severe AR is seen in 11.7% of patients. It increases the risk of all-cause mortality and morbidity. The natural course of AR is it may remain stable, decrease, or increase over time. Echocardiography allows semi-quantitative assessment by assessing the ratio of AR jet width relative to LVOT diameter [Table 5].
| (AR jet width/LVOT diameter) | |
|---|---|
| Mild AR | <25% |
| Moderate AR | 26–64% |
| Severe AR | >65% |
AR: Aortic regurgitation, LVOT: Left ventricular outflow tract
Seller’s angiographic criteria can be used to assess AR severity perioperatively by assessing the degree of the left ventricular opacification.
Echocardiographic grading of AR
Paravalvular AR (PAR)
The prevalence of post-TAVR paravalvular regurgitation varies from 7% to 40% (24). 12% of patients have > moderate PAR post-TAVR.[24]
A meta-analysis which included around 15,000 patients has shown that > moderate Paravalvular leak (PVL) was associated with a two-fold increase in overall all-cause mortality.[25] Newer valve systems are associated with reduced PVL risk due to sealing skirt and valve deployment accuracy.
Semiquantitative parameters such as regurgitant jet width, vena contracta, or pressure half time are less useful for assessment of PAR. PAR jets are usually eccentric and can be multiple [Table 6].
| The proportion of the circumference of the prosthesis covered by the PAR jet in the short-axis view | |
|---|---|
| Mild PAR | <10% |
| Moderate PAR | 10-29% |
| Severe PAR | >30% |
PAR: Paravalvular aortic regurgitation
Echocardiography assessment
Predictors of PAR
Bicuspid aortic anatomy
Asymmetrical calcification especially annular or LVOT calcium
Under sizing of the prosthesis,
Malpositioning of the prosthesis
A lower implantation depth and a greater angle between the ascending aorta and LVOT were shown to play a role in predicting PVL in self-expanding valves.
Large and Elliptical aortic annulus.
Lower body mass index, a high baseline mean aortic gradient, lower ejection fraction, a smaller diameter of TAVI, and a self-expandable TAVI device have been found to be predictors of PAR in FRANCE-TAVI registry.[26]
Moderate-to-severe PAR is associated with higher mortality.[25]
Treatment options are based on causal mechanisms. Post-dilatation of THV can be used if THV is under expanded perioperatively. Transcatheter closure with Amplatzer vascular plug (AVP) can be considered. In the international PLUG – in TAVI registry successful PAR closure was done using AVP III and AVP 4 devices in clinically symptomatic cases of severe PAR with heart failure. Success was achieved in 94% of cases. A retrograde approach is utilized with TEE guidance. Arteriovenous loop may be required in select few cases.
3D transesophageal echocardiography allows direct visualization and planimetry of the vena contracta.
Velocity-encoded magnetic resonance imaging (MRI), which allows the direct measurement of blood flow velocity and volume across the valve and calculation of the regurgitant fraction, has gained increasing interest.
CONDUCTION ABNORMALITIES
Conduction abnormalities normalize in more than half of patients in few days. About 6% (4–13%) of patients however require a permanent pacemaker implantation.
A higher prosthesis-to-LVOT diameter ratio and post-dilatation of the THV system were significantly associated with permanent pacemaker (PPM) implantation in self expanding valve (SEV).
High-degree conduction defects are frequently seen with the self-expanding core valve (38–57%) and they often persist at hospital discharge and at 1-year follow-up.
Pre-existing right bundle branch block (RBBB) is a predictor of pacemaker requirement and mortality at follow-up as evidenced in OCEAN TAVI trial.[27]
10–13% of individuals undergoing TAVR have preexisting left bundle branch block (LBBB). The mere presence of LBBB has not been correlated with PPM requirement. A meta-analysis has shown that new-onset LBBB increases the risk of cardiac death.[28]
New-onset conduction disturbances hinder the recovery of ejection fraction and left ventricular remodeling.[29,30]
Post-TAVR increase in the PR interval was found to be a predictor of late pacemaker implantation in a study by Mangieri et al.[31]
Continuous electrocardiogram (ECG) monitoring is recommended for a minimum of 48 h before being discharged for all patients who exhibit a new LBBB
The criteria for long-term follow-up remain somewhat unclear; however, it is essential to perform an ECG at each follow-up visit. ECG, Holter monitoring, and external loop recorders can be considered in the presence of conduction disturbances.
POST-TAVR LATE STROKE
Ischemic stroke may occur perioperatively or during follow-up. In the SWISS-TAVI registry of 11,957 patients,[32,33] the 30-day cumulative incidence of stroke was 3.0%. About 69% of strokes occurred within 48 h after TAVR. The incidence at 1 year was 4.3% and increased to 7.8% at 5 years. The 30-day incidence of stroke was higher in TAVR patients as compared with medical and surgical ones in the PARTNER trial.
TAVR versus medical therapy 6.7% versus 1.7% (P = 0.03);
TAVR versus SAVR in 5.5% versus 2.4% (P = 0.04).
Late strokes are of thromboembolic origin. It could be due to concurrent AF or due to subclinical thrombosis.
Predictors
Aortic vasculopathy
Prior stroke,
Peripheral vascular disease and
Permanent AF.
LATE EMBOLIZATION OF PROSTHETIC VALVE (LPE)
It is of very rare occurrence. Embolization is usually to left ventricle in 89% of cases, and rarely to the ascending aorta.[34,35]
Risk factors for LPE are as follows:
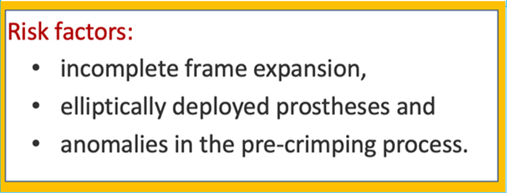
Prosthesis under-sizing, or under-expansion due to aortic root calcification,
Unstable prosthetic positioning,
Lower implant depth into the LVOT,
Insufficient prosthesis anchoring due to large annular calcification,
Bicuspid valve anatomy,
Pre-existing mitral prosthetic valve,
-
Basal septal bulging.
The timing of LPE varies from as early as 2 days to more than a year in its occurrence. It results in acute left ventricular failure and/or cardiogenic shock and mostly patients do not survive.
Moderate paravalvular regurgitation in conjunction with high transvalvular gradients should arouse the suspicion of probable LPE. Emergent surgery is the treatment of choice.
PROSTHESIS COMPRESSION
-
It consists of mechanical deformation of the prosthesis due to external chest compression after attempts at cardiopulmonary resuscitation (CPR). The rarely reported cases[36] have been with the BEV.
It should always be looked for after CPR in patients with THV with BEV. Reported cases are with Sapien valve[37-39] as Stainless steel and Cobalt chromium (XT) do not have shape memory. It is therefore pertinent to give chest compressions in the left hemithorax than the conventional site to avoid this rare but albeit potential complication.
One potential treatment option for prosthetic compression after resuscitation, although reported in only a single case, may involve the re-dilatation of the prosthesis.
PROSTHESIS DURABILITY
Theoretically, the durability of a TAVR prosthesis should be comparable to that of a surgical bio-prosthesis. Durability of the valve is a function of time lapsed after implantation of the valve and the age of the patient at the time of implantation. Durability also varies depending on whether patients are at low-risk, intermediate-risk, or high-risk category.
In a published series, only 3.4% of patients developed a moderate stenosis at 5-year follow-up. The Nordic aortic intervention trial[40] was the first to randomize patients of low risk to TAVR versus SAVR. STS score was 3 ± 1.7%. The patient’s mean age was 79 years and showed no difference in 5-year follow-up for composite outcome for TAVR and SAVR. Greater valvular regurgitation and pacemaker requirement were noted in the TAVR group whereas risk of bioprosthetic failure was similar. No randomized data exists for high-risk elderly TAVR patients as the valves tend to outlive the patient.
POST-TAVR SURVIVAL
The overall survival of TAVR population in the Denmark registry involving 2670 patients majority of whom had self-expanding valve was 58% at 5 years and 20% at 10 years.[41] TAVR in low-risk patients data will help us understand the survival rates in the future.
TIMING OF NON-CARDIAC SURGERY FOLLOWING TAVR
In a cohort study involving 300 patients who underwent TAVR, it was observed that these patients are able to safely undergo non-cardiac surgery shortly after the TAVR procedure when necessary. Patients with moderate-to-severe paravalvular regurgitation and patient-prosthetic mismatch had increased adverse events.[42]
CAN A PATIENT WITH TAVR UNDERGO MRI?
All prosthetic heart valves, mechanical or bioprosthetic, and all co ronary stents are considered safe in the magnetic resonance (MR) environment at field strengths of up to 1.5 Tesla[43] regardless of the value of the spatial gradient magnetic field. It is also prudent to read the package insert label regarding the safety of the device with respect to MRI.
The MRI examination must be performed using the following parameters:[44]
Whole body averaged specific absorption rate of 2 W/kg, operating in the normal operating mode of the MR system
Maximum imaging time of not more than 15 min per pulse sequence (multiple sequences per patient are allowed).
CONCLUSION
Post TAVR care needs to be regularly monitored for achieving long term success. It is important to be aware of the challenges that can arise and ways to tackle them.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- TAVR-associated Prosthetic Valve Infective Endocarditis: Results of a Large, Multicenter Registry. J Am Coll Cardiol. 2014;64:2176-8.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter Heart Valve Failure: A Systematic Review. Eur Heart J. 2015;36:1306-27.
- [CrossRef] [PubMed] [Google Scholar]
- Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA. 2016;316:1083-92.
- [CrossRef] [PubMed] [Google Scholar]
- 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS) the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075-128.
- [CrossRef] [PubMed] [Google Scholar]
- Two-Year Outcomes after Transcatheter or Surgical Aortic-valve Replacement. N Engl J Med. 2012;366:1686-95.
- [CrossRef] [PubMed] [Google Scholar]
- Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438-54.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical or Symptomatic Leaflet Thrombosis Following Transcatheter Aortic Valve Replacement: Insights from the U.S. FDA MAUDE Database. Struct Heart. 2017;1:256-64.
- [CrossRef] [Google Scholar]
- Subclinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Valves: PARTNER 3 Cardiac Computed Tomography Sub Study. J Am Coll Cardiol. 2020;75:3003-15.
- [CrossRef] [PubMed] [Google Scholar]
- Registry of Transcatheter Aortic-valve Implantation in High-risk Patients. N Engl J Med. 2012;366:1705-15.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter Aortic-valve Replacement for Inoperable Severe Aortic Stenosis. N Engl J Med. 2012;366:1696-704.
- [CrossRef] [PubMed] [Google Scholar]
- Natural History of Leaflet Thrombosis After Transcatheter Aortic Valve Replacement: A 5-Year Follow-Up Study. J Am Heart Assoc. 2022;11:e026334.
- [CrossRef] [PubMed] [Google Scholar]
- Apixaban and Valve Thrombosis After Transcatheter Aortic Valve Replacement: The ATLANTIS-4D-CT Randomized Clinical Trial Substudy. JACC Cardiovasc Interv. 2022;15:1794-804.
- [CrossRef] [PubMed] [Google Scholar]
- Antithrombotic Therapy After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2021;14:1688-703.
- [CrossRef] [PubMed] [Google Scholar]
- Antithrombotic Therapy Following Transcatheter Aortic Valve Replacement. J Clin Med. 2022;11:2190.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:2523-33.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and Outcomes of Acute Coronary Syndrome After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:938-50.
- [CrossRef] [Google Scholar]
- Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2020;13:e008620.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary Access After TAVR. JACC Cardiovasc Interv. 2020;13:693-705.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc Interv. 2020;13:2542-55.
- [CrossRef] [PubMed] [Google Scholar]
- Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: Results From the Multicenter International BASILICA Registry. JACC Cardiovasc Interv. 2021;14:941-8.
- [CrossRef] [PubMed] [Google Scholar]
- Procedural and One-year Outcomes of the BASILICA Technique in Europe: The Multicentre EURO-BASILICA Registry. EuroIntervention. 2023;19:e432-41.
- [CrossRef] [PubMed] [Google Scholar]
- Glutaraldehyde-fixed Bioprosthetic Heart Valve Conduits Calcify and Fail from Xenograft Rejection. Circulation. 2006;114:318-27.
- [CrossRef] [PubMed] [Google Scholar]
- Late Incidence and Determinants of Reoperation in Patients with Prosthetic Heart Valves. Eur J Cardiothorac Surg. 2004;25:364-70.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodynamic Assessment of Aortic Regurgitation after Transcatheter Aortic Valve Replacement: The Diastolic Pressure-time Index. JACC Cardiovasc Interv. 2016;9:1061-8.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of Paravalvular Regurgitation on the Mid-term Outcome after Transcatheter and Surgical Aortic Valve Replacement. Eur J Cardiothorac Surg. 2020;58:1145-52.
- [CrossRef] [PubMed] [Google Scholar]
- Long-Term Prognosis Value of Paravalvular Leak and Patient-Prosthesis Mismatch following Transcatheter Aortic Valve Implantation: Insight from the France-TAVI Registry. J Clin Med. 2022;11:6117.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-Existing Right Bundle Branch Block Increases Risk for Death After Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve. JACC Cardiovasc Interv. 2016;9:2210-6.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of New-onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ Cardiovasc Interv. 2016;9:e003635.
- [CrossRef] [PubMed] [Google Scholar]
- Impact on Left Ventricular Function and Remodeling and on 1-year Outcome in Patients with Left Bundle Branch Block after Transcatheter Aortic Valve Implantation. Am J Cardiol. 2015;116:125-31.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of Procedure-related Conduction Disturbances After Transcatheter Aortic Valve Implantation on Myocardial Performance and Survival Evaluated by Conventional and Speckle Tracking Echocardiography. Echocardiogr Mt Kisco N. 2018;35:621-31.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of Advanced Conduction Disturbances Requiring a Late (=48 H) Permanent Pacemaker Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:1519-26.
- [CrossRef] [PubMed] [Google Scholar]
- Long-Term Risk of Stroke After Transcatheter Aortic Valve Replacement: Insights From the SwissTAVI Registry. JACC Cardiovasc Interv. 2023;16:2986-96.
- [CrossRef] [PubMed] [Google Scholar]
- Late Stroke after Transcatheter Aortic Valve Replacement: A Nationwide Study. Sci Rep. 2021;11:9593.
- [CrossRef] [PubMed] [Google Scholar]
- Severe Valve Deformation Following Cardiopulmonary Resuscitation in a Patient with a Transcatheter Aortic Valve. JACC Cardiovasc Interv. 2015;8:498-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ventricular Embolization of Edwards SAPIEN Prosthesis Following Transcatheter Aortic Valve Implantation. J Invasive Cardiol. 2012;24:537-8.
- [Google Scholar]
- Late Downward Dislocation of a Balloon Expandable Valve into the Left Ventricular Outflow Tract Following Transfemoral Transcatheter Aortic Valve Implantation. Circ J. 2013;77:1345-7.
- [CrossRef] [PubMed] [Google Scholar]
- Transapical Valve Implantation and Resuscitation: Risk of Valve Destruction. Ann Thorac Surg. 2011;92:1909-10.
- [CrossRef] [PubMed] [Google Scholar]
- Valve Prosthesis Distortion after Cardiac Compression in a Patient Who Underwent Transcatheter Aortic Valve Implantation (TAVI) Catheter Cardiovasc Interv. 2012;83:E165-7.
- [CrossRef] [Google Scholar]
- Deformation of a Transapical Aortic Valve after Cardiopulmonary Resuscitation: A Potential Risk of Stainless Steel Stents. J Am Coll Cardiol. 2012;60:1838.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter or Surgical Aortic Valve Implantation: 10-year Outcomes of the NOTION Trial. Eur Heart J. 2024;45:1116-24.
- [CrossRef] [PubMed] [Google Scholar]
- Life Expectancy of Patients with a Transcatheter Aortic Valve and the Implications for Long-term Valve Durability Data Collection. EuroIntervention. 2023;18:996-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk and Timing of Noncardiac Surgery After Transcatheter Aortic Valve Implantation. JAMA Netw Open. 2022;5:e2220689.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of Magnetic Resonance Imaging in Patients with Cardiovascular Devices: An American Heart Association Scientific Statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: Endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878-91.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic Resonance Imaging is a Safe Technique in Patients with Prosthetic Heart Valves and Coronary Stents. Hellenic J Cardiol. 2019;60:38-9.
- [CrossRef] [PubMed] [Google Scholar]







