Translate this page into:
To Compare the Efficacy of Rivaroxaban with Nicoumolone in the Management of Patients with Intraventricular Thrombus
*Corresponding author: Boochibabu Mannuva, Department of Cardiology, Guntur Medical College, Guntur, Andhra Pradesh, India. e.mbb99@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bose Yannam JC, Battula N, Annangi SS, Mannuva B, Nathani S, Betham R, et al. To Compare the Efficacy of Rivaroxaban with Nicoumolone in the Management of Patients with Intraventricular Thrombus. Indian J Cardiovasc Dis W omen. 2025;10:30-5. doi: 10.25259/IJCDW_28_2024
Abstract
Objectives:
Left ventricular thrombus (LVT) poses serious risks, including stroke and systemic embolism. Anticoagulant therapy is crucial, yet uncertainty persists in optimal management. This study aims to compare nicoumalone and rivaroxaban for LVT, assessing efficacy, safety, and monitoring considerations to inform clinical decision-making.
Materials and Methods:
This prospective and open-label trial compared rivaroxaban and nicoumalone efficacy in managing LVT over a 1-year period (September 2021–September 2022). Thirty-one patients from Government General Hospital, Guntur, meeting inclusion criteria were enrolled. Data were collected through interviews and echocardiography. Primary outcomes included thrombus resolution and recurrence; secondary outcomes encompassed adverse effects such as bleeding and stroke. Standard dosing and monitoring protocols were followed for each intervention.
Results:
In this study, both nicoumalone and rivaroxaban groups exhibited a reduction in LVT size over time, although not statistically significant (P > 0.05). Complete resolution was achieved in 70.0% of nicoumalone patients and 81.8% of rivaroxaban patients, with no significant difference (P = 0.472). However, rivaroxaban group patients achieved complete resolution significantly faster (176.67 ± 43.589 days) compared to nicoumalone group patients (271.67 ± 115.24 days) (P = 0.028). Notably, one nicoumalone patient experienced LVT recurrence post-anticoagulation cessation.
Conclusion:
Rivaroxaban was discovered to be non-inferior to nicoumolone in terms of efficacy and safety in patients with LVT.
Keywords
Left ventricular clotrivaroxaban
NOACs
Nicoumolone
Anticoagulation
Rivaroxaban
ABSTRACT IMAGE
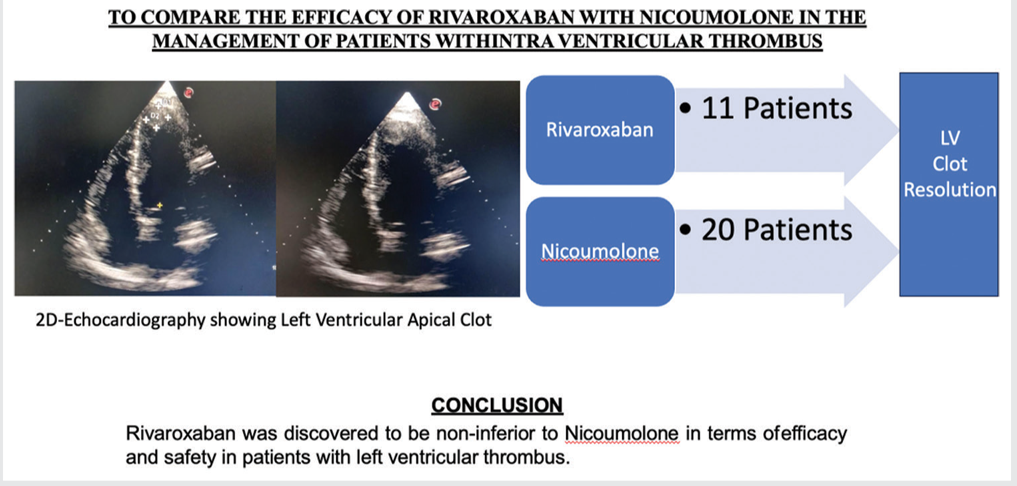
LV: Left ventricle
INTRODUCTION
Left ventricular thrombus (LVT) is a medical condition where the blood clot is formed as a result of blood stasis within the left ventricle. It is a significant medical problem with potential complications, including stroke, systemic embolism, and myocardial infarction. It can occur in individuals with various underlying cardiovascular conditions, such as cardiomyopathy (dilated, infiltrative, and restrictive), ischemic heart disease, and atrial fibrillation.[1-3]
The LVT formation typically occurs that this stagnant blood flow can be attributed to factors such as impaired cardiac function, abnormal heart rhythms, or structural abnormalities of the heart.[4]
LVT poses a considerable risk to affected individuals due to its potential to embolize, leading to severe complications such as stroke and gangrene of foot. Thus, prompt diagnosis of LVT and appropriate management are essential to prevent these potentially life-threatening consequences.[5,6]
Diagnostic techniques such as echocardiography, including transthoracic echocardiography (TTE) and transesophageal echocardiography, may play an important role in identifying LVT. These diagnostic modalities can provide detailed visualization of the heart chambers, allowing the detection and characterization of thrombi within the left ventricle.[1,7,8]
The LVT treatment strategy involves the use of various anticoagulant drugs and interventions aimed at preventing formation of clot and embolism risk reduction. Anticoagulant medications, such as oral anticoagulants or injectables such as heparin, may help in the prevention of the formation of a clot and promote the dissolution of existing thrombus. In some cases, surgical removal of the thrombus may be necessary, particularly if it poses a high risk of embolization or fails to respond to medical management.
Over the years, the field of anticoagulation has witnessed remarkable advancements in drug development and treatment strategies. Traditional anticoagulants, for example, warfarin, have been commonly used for decades, while newer direct oral anticoagulants (DOACs) have become widely recognized and favored due to their predictable pharmacokinetics, fewer drug interactions, and ease of use.[8,9]
The anticoagulant choice and the duration of therapy depend on several factors, which include the underlying specific medical condition, the individual patient’s risk factors, and the presence of other comorbidities. It is very important to strike a balance between the benefits of anticoagulation in preventing clotting-related complications and the potential risks of bleeding associated with these medications.[8,9]
This study aims to compare novel oral anticoagulant (NOAC) in LVT management, comparing the clinical efficacy, monitoring considerations, and potential complications.
MATERIALS AND METHODS
Study design
This study is an observational, prospective, open-label, and intervention efficacy trial.
Duration of the study
The study period duration was between September 2021 and September 2022.
Sample size
This sample was 31.
Source of data
The patient data were collected from outpatient department (OPD) patients and admitted patients in Cardiology Department at the Government General Hospital, Guntur. The study was initiated after the Hospital Ethics Committee approval.
Inclusion criteria
All patients more than 18 years age and less than 80 years
Acute myocardial infarction/ischemic cardiomyopathy/dilated cardiomyopathy
With diagnosis of LVT.
Exclusion criteria
Patients below 18 years or above 80 years
Pregnancy
Patients with valvular heart disease
Patients with arrhythmias
Patients with pacemakers or implanted cardiac defibrillators in situ
Liver dysfunction
Severe renal dysfunction
Bleeding
Hematological disorders
Malignancy
Patients with contraindications to anticoagulant therapy, such as active bleeding, bleeding disorders, or known hypersensitivity to either rivaroxaban or warfarin.
Study intervention
The study compared the efficacy and adverse effects of two anticoagulant drugs: Rivaroxaban (DOAC) and nicoumalone (Vitamin K antagonist [VKA]). Patients meeting the inclusion criteria were assigned to one of the two treatment groups based on their preference and the treating physician’s recommendation. The intervention was administered as per the standard dosing and monitoring guidelines for each drug.
Data collection methods
The required patient data were obtained from patient interview, including medical history, demographic information, and relevant clinical parameters. Information on adverse effects such as bleeding events and stroke was recorded during follow-up visits. Trained study personnel collected the data using standardized data collection forms. TTE was used to assess for LVT. The left ventricular cavity and wall motion in each view for detection of an abnormal mass or thrombus was carefully observed [Figures 1 and 2].
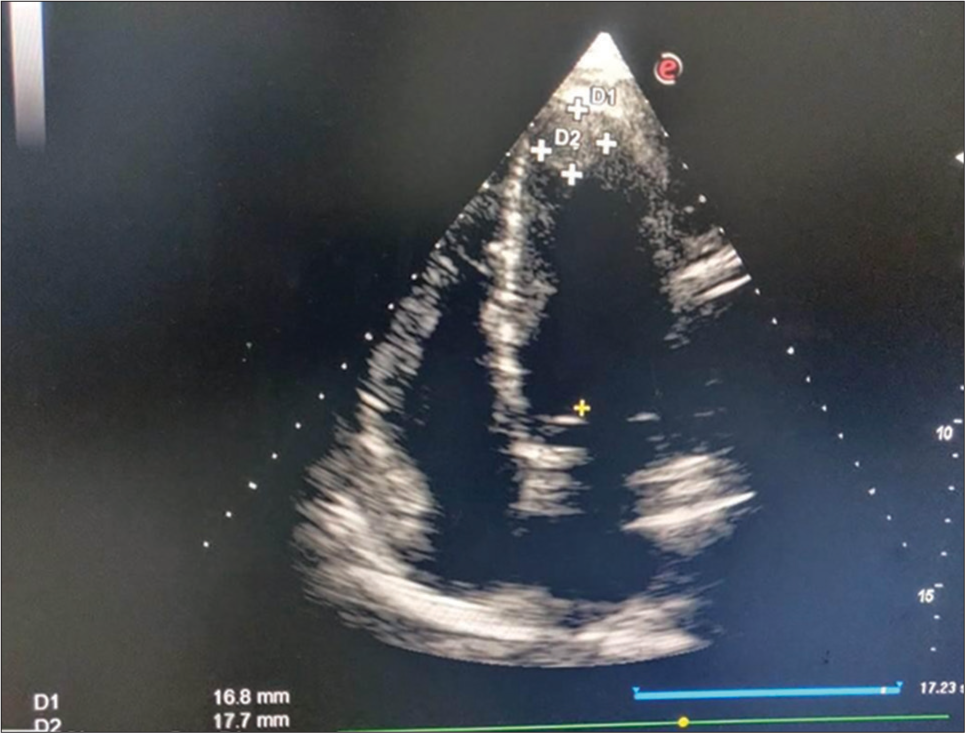
- This picture shows two-dimensional echocardiography (apical four-chamber view) with the left ventricular thrombus attached to the apex and measured 17 × 18 mm.
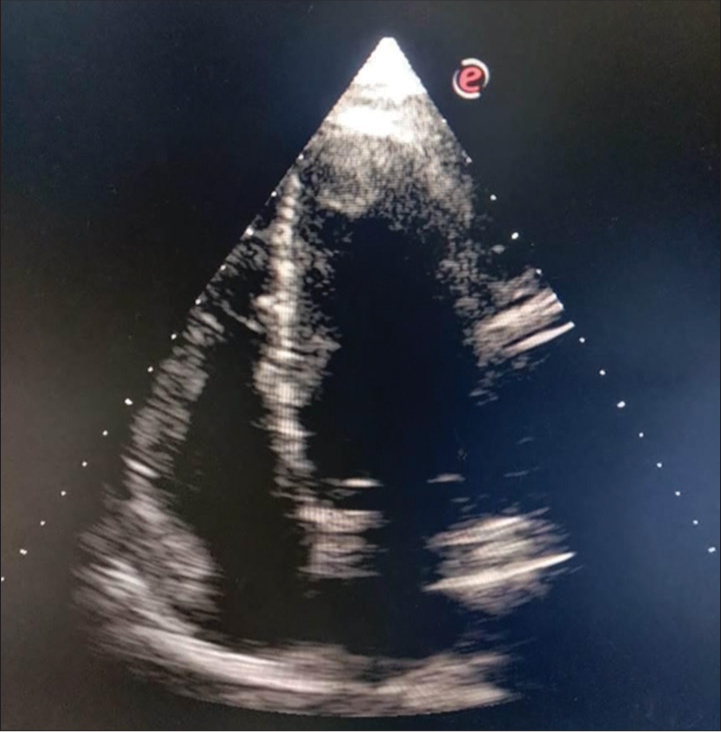
- This picture shows two-dimensional echocardiography (apical four-chamber view) with the left ventricular thrombus attached to the apex.
Outcome measures
The primary outcome measure was the efficacy of rivaroxaban compared to nicoumalone in the management of LVT. This was assessed by monitoring the thrombus resolution and any recurrence of thrombus during the study period. Secondary outcome measures included the incidence of adverse effects such as bleeding events and stroke in both treatment groups.
Statistical analysis
Descriptive statistical analysis was done to summarize the baseline characteristics in the study population. The incidence of thrombus resolution and adverse events were calculated and compared between the rivaroxaban and nicoumalone groups. Inferential statistical tests, such as Chi-square test or Fisher’s exact test, were utilized to assess the significance of differences between the two treatment groups.
RESULTS
In this study, 31 patients with clinical and echocardiographic evidence of LVT were enrolled from the cardiology OPD, emergency department, and inpatient wards. Patients were divided into two groups based on their chosen anticoagulant: Nicoumalone (VKA) or rivaroxaban (DOAC) [Table 1].
| Drug name | No. of patients | (%) of patients |
|---|---|---|
| Nicoumalone | 20 | 64.5 |
| Rivaroxaban | 11 | 35.5 |
Twenty patients opted for nicoumalone, while 11 chose rivaroxaban. The mean age of the cohort was 52.39 ± 8.697 years, with 26 men (83.9%) and 5 women (16.1%). Among them, 64.5% had acute coronary syndrome (ACS), while 35.5% had dilated cardiomyopathy (DCMP).
In our study cohort, comprising 31 patients with LVT, various demographic and clinical characteristics were observed. Among the patients, 9 (29%) had diabetes mellitus, while 10 (32.3%) had hypertension. Smoking was prevalent in 17 (54.8%) patients, and alcohol consumption was reported in 14 (45.2%) individuals [Table 2].
| Nicoumalone group n(%) (20 patients) | Rivaroxaban group n(%) (11 patients) | P-value | |
|---|---|---|---|
| Age (years) | 53.70±9.8 | 50.00±5.6 | 0.268 |
| Gender (males) | 16 (80) | 10 (90.9) | 0.429 |
| Diabetes | 6 (30.0) | 3 (27.3) | 0.873 |
| Hypertension | 8 (40.0) | 2 (18.2) | 0.214 |
| Smoking | 10 (50.0) | 7 (63.6) | 0.465 |
| Alcohol | 8 (40.0) | 6 (54.5) | 0.436 |
| Etiology (ACS/DCMP) | 14 (70)/6 (30) | 6 (54.5)/5 (45.5) | 0.390 |
| Echocardiographic parameters | |||
| LVIDd (mm) | 52.35±6.40 | 56.64±9.00 | 0.134 |
| LVIDs (mm) | 41.90±6.21 | 46.82±8.83 | 0.080 |
| EF % | 38.30±7.40 | 35.91±7.09 | 0.390 |
ACS: Acute coronary syndrome, DCMP: Dilated cardiomyopathy, LVIDd: Left ventricular internal dimension at end-diastole, LVIDs: Left ventricular internal dimension at end-systole, EF: Ejection fraction
Gender distribution indicated that 4 (20%) nicoumalone patients and 1 (9.1%) rivaroxaban patient were women. The mean age in the nicoumalone group was 53.70 ± 9.8 years, slightly higher than the rivaroxaban group’s mean age of 50.00 ± 5.6 years. Diabetic patients accounted for 6 out of 20 (30.0%) in the nicoumalone group and 3 out of 11 (27.3%) in the rivaroxaban group. Hypertension was present in 8 (40.0%) nicoumalone patients and 2 (18.2%) rivaroxaban patients. Smoking prevalence was similar between the two groups, with 10 (50.0%) nicoumalone patients and 7 (63.6%) rivaroxaban patients being smokers. Alcohol consumption was reported by 8 (40.0%) nicoumalone patients and 7 (63.6%) rivaroxaban patients [Table 2].
Regarding underlying conditions, 14 (70%) nicoumalone patients presented with acute coronary syndrome (ACS), while 6 (30%) presented with DCMP. In the rivaroxaban group, 6 (54.5%) patients had ACS, and 5 (45.5%) had DCMP.
TTE was performed for all patients to monitor LVT size and resolution [Tables 3 and 4]. While the reduction in LVT size over time was not statistically significant (P = not significant), complete resolution was achieved by 14 (70.0%) nicoumalone patients and 9 (81.8%) rivaroxaban patients (P = 0.49). The time to complete resolution differed significantly between the two groups (P = 0.028), with nicoumalone patients requiring 271.67 ± 115.24 days compared to 176.67 ± 43.589 days for rivaroxaban patients. Notably, one nicoumalone patient experienced recurrence after discontinuation of anticoagulation.
| LVT size (mm2) | Nicoumalone group | Rivaroxaban group | P-value |
|---|---|---|---|
| Entry | 303.30±213.26 | 229.40±91.16 | 0.306 |
| At 3 months | 191.40±148.23 | 238.75±99.60 | 0.451 |
| At 6months | 131.68±118.8 | 74.09±144.582 | 0.247 |
| At 9 months | 68.00±75.982 | 19.00±60.083 | 0,127 |
| At 1 year | 55.20±87.013 | 32.55±72.576 | 0.490 |
LVT: Left ventricular thrombus
| Clot resolution | Nicoumalone group (%) | Rivaroxaban group (%) | P-value |
|---|---|---|---|
| Complete resolution | 14 (70.0) | 9 (81.8) | 0.472 |
| Residual thrombus | 6 (30.0) | 2 (18.2) | |
| Number of days | 271.67±115.24 | 176.67±43.589 | 0.028 |
DISCUSSION
This study represents a pioneering effort in India, examining the efficacy and safety of rivaroxaban (DOAC) compared to nicoumalone, a VKA, in managing LVT. Our investigation sheds light on the clinical intricacies and therapeutic outcomes of LVT patients subjected to distinct anticoagulant regimens. While both nicoumalone and rivaroxaban demonstrated potential in LVT management, with reassuringly low incidences of serious bleeding, our findings did not unveil a significant disparity in thrombus resolution rates between the two drugs (P > 0.05). This outcome indicates that rivaroxaban could be a viable alternative to nicoumalone in LVT treatment, suggesting comparable efficacy and safety profiles between the two agents.
Contrary to our findings, previous meta-analyses, such as the study by Ali et al.,[10] encompassing a broader comparison between warfarin and DOACs, suggested a marginal advantage of DOACs in promoting thrombus resolution. However, our study focused on a specific comparison between nicoumalone and rivaroxaban, providing valuable insights into the relative effectiveness of these agents in LVT management. While our results align with the notion that DOACs may offer similar benefits to warfarin in terms of thrombus resolution and safety outcomes, the direct comparison between nicoumalone and rivaroxaban elucidates their non-inferiority, thereby expanding the armamentarium of anticoagulant options for LVT patients.
It is noteworthy that our study cohort exhibited a relatively low incidence of bleeding complications, with only 12.4% of patients experiencing mild bleeding events, such as petechial rash and ecchymosis. Notably, one patient in the rivaroxaban group experienced per rectal bleeding, which did not require blood transfusion. Importantly, no severe bleeding events, such as intracerebral hemorrhage or intramuscular hematoma necessitating transfusion, were recorded in our sample. These findings underscore the favorable safety profile of both nicoumalone and rivaroxaban in LVT management, reassuring clinicians regarding their use in this clinical context.
In a 2020 retrospective study by Willeford et al., comparing DOACs to warfarin for LVT treatment, 54.3% of patients on warfarin achieved efficacy outcomes compared to 40.9% on DOACs. However, no statistical difference was found between the two groups (P = 0.25). The odds ratio for effectiveness was 0.39 (P = 0.07), suggesting equivalent treatment efficacy between warfarin and DOACs in LVT patients.[11]
However, our study is not without limitations. The single-center design may limit the generalizability of our findings, and the relatively small sample size could restrict statistical power. In addition, the lack of randomization and potential for subjective interpretation of outcomes might introduce bias into our results. Future research endeavors should focus on larger, multicenter studies employing rigorous randomization and blinding techniques to validate our findings and provide robust evidence for clinical decision-making in LVT management.
Our research sheds light on the clinical characteristics and prognoses of LVT patients managed with various anticoagulant regimens. Our preliminary findings imply that both nicoumalone (VKA) and rivaroxaban (NOAC) may be feasible alternatives for anticoagulation in patients with LVT, while more research and follow-up are required to assess long-term effects. Our study’s observation of a low incidence of serious bleeding problems is reassuring.
CONCLUSION
Rivaroxaban was discovered to be non-inferior to nicoumolone in terms of efficacy and safety in patients with LVT.
Ethical approval
The research/study was approved by the Institutional Review Board at Guntur Medical College, approval number 168/2021, dated November 12, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Audio summary available at
Financial support and sponsorship: Nil.
References
- Left Ventricular Thrombus and Subsequent Thromboembolism, Comparison of Anticoagulation, Surgical Removal, and Antiplatelet Agents. J Atheroscler Thromb. 2013;20:73-93.
- [CrossRef] [PubMed] [Google Scholar]
- Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:1010-22.
- [CrossRef] [PubMed] [Google Scholar]
- Left Ventricular Thrombi after STEMI in the Primary PCI Era: A Systematic Review and Meta-analysis. Int J Cardiol. 2016;221:554-9.
- [CrossRef] [PubMed] [Google Scholar]
- 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119-77.
- [CrossRef] [PubMed] [Google Scholar]
- Echocardiography vs. Cardiac Magnetic Resonance Imaging for the Diagnosis of Left Ventricular Thrombus: A Systematic Review. Can J Cardiol. 2015;31:785-91.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of Direct Acting Oral Anticoagulants in Treatment of Left Ventricular Thrombus. Am J Cardiol. 2019;124:367-72.
- [CrossRef] [PubMed] [Google Scholar]
- Switching from Warfarin to Direct-acting Oral Anticoagulants: It Is Time to Move Forward! Egypt Heart J. 2022;74:18.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Effectiveness of Direct Oral Anticoagulants and Warfarin for the Treatment of Left Ventricular Thrombus. J Thromb Thrombolysis. 2021;52:517-22.
- [CrossRef] [PubMed] [Google Scholar]
- Off-label Use of Direct Oral Anticoagulants Compared With Warfarin for Left Ventricular Thrombi. JAMA Cardiol. 2020;5:685-92.
- [CrossRef] [PubMed] [Google Scholar]
- Direct Oral Anticoagulant Use In left Ventricular Thrombus. Thromb J. 2020;18:29.
- [CrossRef] [PubMed] [Google Scholar]
- Direct Oral Anticoagulants Versus Warfarin in the Treatment of Left Ventricular Thrombus. Ann Pharmacother. 2021;55:839-45.
- [CrossRef] [PubMed] [Google Scholar]







