Translate this page into:
Study of Acute Coronary Syndrome in Premenopausal Women in Correlation with Sex Hormones
*Corresponding author: Veena Nanjappa, Department of Cardiology, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Mysore, Karnataka, India. veenananjappa@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Nanjappa V, Raveesh H, Kuldeep A, Sadanand KS, Manjunath CN. Study of acute coronary syndrome in premenopausal women in correlation with sex hormones. Indian J Cardiovasc Dis Women 2022;7:204-9.
Abstract
Objectives:
Higher testosterone and lower Estrogen levels are associated with cardiovascular disease in women. However, studies on endogenous sex hormones and acute coronary syndrome (ACS) in pre-menopausal women are sparse.
Material and Methods:
We studied 50 pre-menopausal women presenting with ACS and age-matched controls who consented to the study with Testosterone, Estradiol, and Sex hormone-binding globulin (SHBG) levels at baseline. They were clinically followed up for 6 months duration.
Results:
The mean age was 37.42 ± 5.7 years. 48% patients were obese. The mean body mass index was 27.53 ± 5.41 kg/m2. Hypertension followed by Diabetes was the most common risk factor. 14% had family history of coronary artery disease (CAD). 24% had atypical chest pain at presentation. Anterior wall ST elevation myocardial infarction was the most common presentation. Single-vessel disease was seen in 38%; 24% had Non-Obstructive CAD. Ratios of Bioavailable Testosterone: Estradiol, Estradiol: Low-density Lipoprotein (LDL), Estradiol: High-density Lipoprotein, SHBG: LDL, and SHBG: HbA1c were analyzed in cases and controls and were not found to be significantly associated.
Conclusion:
Endogenous sex hormones were not found to be significantly associated with ACS in premenopausal women.
Keywords
Acute coronary syndrome
Endogenous sex hormone
Premenopausal women
ABSTRACT IMAGE

INTRODUCTION
A leading cause of death is coronary artery disease (CAD) in women.[1] There are specific differences in the prevalence of CAD with respect to gender. CAD risk increases in women after menopause.[2] Higher androgen and lower levels of endogenous estrogen that occurs after menopause may result in an increased CAD risk in postmenopausal women.[2,3] The altered hormonal milieu in postmenopausal women is associated with CAD risk factors, such as insulin resistance, high C-reactive protein, and high blood pressure.[5-7] In MESA study, an elevated risk for heart failure and cardiovascular disease (CVD) was seen in patients with a higher ratio of serum testosterone to estradiol.[4]
Higher risk for CAD was seen in women with higher total testosterone levels and higher estradiol levels were associated with a lower risk of CAD. Women’s Health Initiative, Heart and Estrogen-Progestin Replacement Study 2 (HERS2), and HERS studies do not support the beneficial vascular effects of hormone replacement therapy in postmenopausal women.[8,9] Receptors for estrogen, progesterone, and testosterone are expressed in endothelium and vascular smooth muscle of multiple vascular systems.[10,11] It is one of the proposed mechanism responsible for gender difference in vascular tone.
Increased serum prolactin levels are associated with major depressive disorder especially in women. There is an increased risk of developing cardiometabolic disease in major depressive disorders. Physiologically, prolactin has a role in the regulation of stress, anxiety, and weight gain. Dysregulation of neuroendocrine axis in major depressive illness may extend to this hormone.[12]
Very few studies have examined the role of endogenous sex hormones and CAD in the premenopausal age group. There is an increasing trend of premenopausal women presenting with acute coronary syndrome (ACS). This study was funded by Rajiv Gandhi University of Health Sciences, Bengaluru.
MATERIALS AND METHODS
Fifty consecutive patients with ACS in the premenopausal age group who consented to the study were included in the study. Age-matched controls were taken. Clinical, demographic, and treatment details were recorded. Three months clinical and telephonic follow-up was done post-discharge. The concentrations of sex hormones were measured from fasting serum samples drawn at the baseline between 7:30 AM and 10:30 AM. It was stored at −70°C until analysis. Radioimmunoassay kits were used to measure total testosterone levels. Sex hormone-binding globulin (SHBG) was measured using a chemiluminescent enzyme immunometric assay. Estradiol was measured using an ultra-sensitive radioimmunoassay kit. Concentrations of bioavailable testosterone (the sum of SHBG-bound and albumin-bound Testosterone) and free testosterone (reported as a percentage of total Testosterone) were calculated.[14] Total testosterone/estradiol ratio was calculated for each participant. Serum prolactin levels were assayed.
Statistical method used
The data were analyzed using Microsoft Excel and R-4.1.0 software. All the tests of significance were carried out at 5% level of significance. The descriptive results displayed as subgroups of cases and controls. The numerical data are presented as mean and standard deviations. All the categorical data are presented in the study as frequency and percentages. Descriptive statistics is used for frequency tables, summary statistics and inferential statistics for independent sample t-test were used.
RESULTS
Most patients were in the 31–40 years age group [Table 1]. The mean age was 37.42 ± 5.7 years. About 48% were obese. The mean body mass index was 27.53 ± 5.41 kg/m2. There were no patients in study group who consumed alcohol or tobacco in any form. Hypertension followed by diabetes was the most common risk factors. About 14% had a family history of CAD. About 24% had atypical chest pain at presentation and 22% had presyncopal symptoms. ST-segment elevation myocardial infarction (STEMI) was the most frequent presentation with anterior wall MI being the most common [Figure 1]. About 48% had mild LV systolic dysfunction and 84% of patients were in Killip’s class I at presentation. About 90% of patients underwent angiogram; single-vessel disease was seen in 38% and left anterior descending artery was the most common vessel involved; and 24% had non-obstructive CAD.
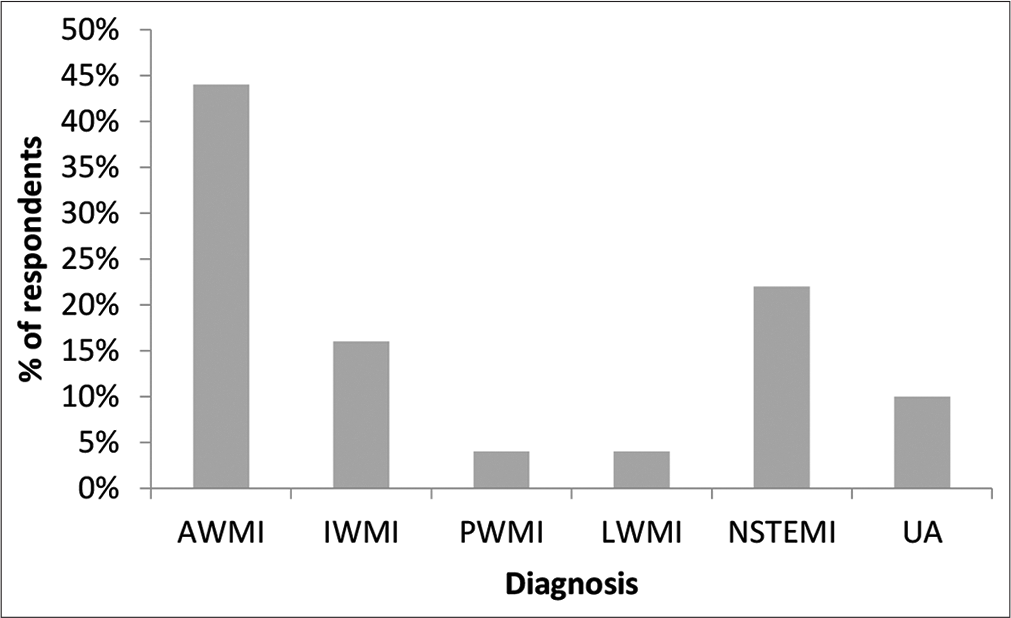
- Percentage distribution of type of MI in the study group.
| Variables | n (%) |
|---|---|
| Age | |
| 20–30 years | 2 (4%) |
| 31–40 years | 26 (52%) |
| 41–50 years | 22 (44%) |
| BMI (kg/m2) | |
| <18.5 | 2 (4%) |
| 18.5–24.9 | 24 (48%) |
| 25–29.9 | 18 (36%) |
| 30–39.9 | 6 (12%) |
| Risk factors | |
| Diabetes | 21 (42%) |
| Hypertension | 26 (52%) |
| Family history | 7 (14%) |
| COPD | 3 (6%) |
| Hypothyroid | 2 (4%) |
| Previous IHD | 15 (30%) |
| Anemia | 9 (18%) |
| Symptoms | |
| Chest pain | 36 (72%) |
| Typical | 24 (48%) |
| Atypical | 12 (24%) |
| Dyspnea | 20 (40%) |
| Fatigue | 38 (76%) |
| Palpitation | 3 (6%) |
| PND | 9 (18%) |
| Orthopnea | 9 (18%) |
| Back ache | 15 (30%) |
| Pre-syncope/syncope | 11 (22%) |
| Diagnosis | |
| AWMI | 22 (44%) |
| IWMI | 8 (16%) |
| PWMI | 2 (4%) |
| LWMI | 2 (4%) |
| NSTEMI | 11 (22%) |
| Unstable angina | 5 (10%) |
| EF% | |
| <30 Severe | 0 |
| 30–40 Moderate | 2 (8%) |
| 40–50 Mild | 24 (48%) |
| >50 Adequate | 22 (44%) |
| KILLIPs class | |
| Class I | 42 (84%) |
| Class II | 6 (12%) |
| Class III | 2 (4%) |
| Class IV | 0 |
| CAG | 45 (90%) |
| SVD | 19 (38%) |
| DVD | 10 (20%) |
| TVD | 4 (8%) |
| Non-obstructive CAD | 12 (24%) |
| PTCA | 20 (40%) |
About 26% had pre-diabetes and 50% had diabetes in the cases group [Figure 2].
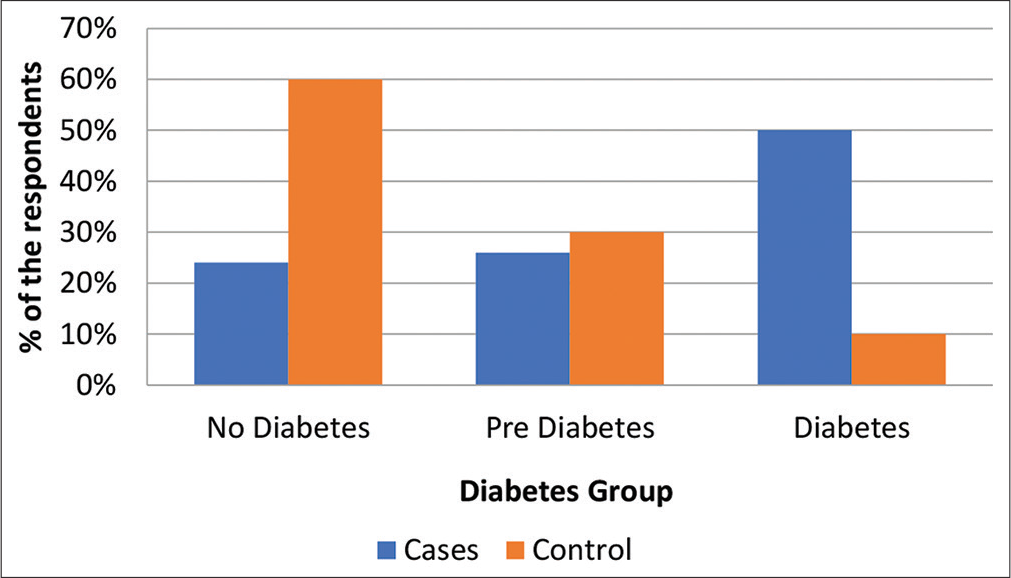
- Percentage distribution of patients based on glycemic status.
About 56% had anemia and 98% had low-density lipoprotein (LDL) levels greater than 70 mg/dl. The mean LDL was 104.44 ± 18.59 mg/dl. The mean high-density lipoprotein (HDL) was 39.32 ± 4.37 mg/dl. About 78% had high triglyceride levels. The mean triglycerides were 175.28 ± 74.79 mg/dl. There were marginally higher levels of serum prolactin levels in cases than in controls – 17.78 ng/ml versus 14.85ng/ml. The total testosterone to estradiol ratio showed an inverse relationship in cases with a positive trend but was not statistically significant. When bioavailable testosterone: estradiol levels were analyzed, it was not found to differ in the two groups. Ratios of estradiol with LDL and HDL were not significant [Table 2]. The relationship between SHBG and HBA1c levels was not found to be significant [Table 3].
| Variables | Group | n | Mean | Standard deviation | t-test value | df | P-value | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Total Testosterone: Estradiol | Cases | 50 | 0.2992 | 0.2264 | 1.794 | 98 | 0.076 | Not Significant |
| Control | 50 | 0.4072 | 0.3698 | |||||
| Bioavailable testosterone: Estradiol | Cases | 50 | 0.1354 | 0.1276 | 1.156 | 98 | 0.251 | Not Significant |
| Control | 50 | 0.1699 | 0.1680 | |||||
| Estradiol: LDL | Cases | 50 | 0.0013 | 0.0013 | 1.269 | 98 | 0.208 | Not Significant |
| Control | 50 | 0.0017 | 0.0017 | |||||
| Estradiol: HDL | Cases | 50 | 0.0034 | 0.0031 | 1.295 | 98 | 0.198 | Not Significant |
| Control | 50 | 0.0043 | 0.0042 | |||||
| SHBG: LDL | Cases | 50 | 0.4052 | 0.2449 | 0.970 | 98 | 0.335 | Not Significant |
| Control | 50 | 0.4764 | 0.4576 | |||||
| Follicular | Cases | 26 | 81.335 | 12.9603 | 0.553 | 50 | 0.583 | Not Significant |
| Control | 26 | 93.362 | 17.4682 | |||||
| Luteal | Cases | 24 | 101.925 | 83.9084 | 0.591 | 46 | 0.557 | Not Significant |
| Control | 24 | 115.938 | 90.2985 |
SHBG: Sex hormone-binding globulin, LDL: Low-density lipoprotein, HDL: High-density lipoprotein
| Group | n | Correlation value | P-value | Conclusion |
|---|---|---|---|---|
| Combined | 100 | −0.176 | 0.079 | Not significant |
| Cases | 50 | −0.249 | 0.081 | Not significant |
| Control | 50 | −0.11 | 0.447 | Not significant |
Interpretation: Lower degree negative correlation, SHBG: Sex hormone-binding globulin
DISCUSSION
CAD increases exponentially after a woman attains menopause. It is said to be due to the wearing off of the protective effect of endogenous sex hormones.[13] Endogenous sex hormones have both genomic and non-genomic effects on endothelium and vascular smooth muscle. It was shown that women have a higher risk of death and heart failure than men in the 5 years following a STEMI even after accounting for differences in angiographic findings, revascularization, and other confounders in a large-scale cohort study.[14] In our study, there were no deaths documented in-hospital. One patient died on follow-up at 3 months for non-cardiac reasons.
Women with CAD characteristically have a higher prevalence of angina, greater comorbidities, and higher prevalence of non-obstructive CAD on angiography than men with CAD.[14,15] In our study, 42% and 52% had diabetes and hypertension, respectively. Prior ischemic heart disease was seen in 30% of cases. Non-obstructive CAD is known to predominantly affect postmenopausal women,[16] where estrogen is implicated in coronary microvascular dysfunction by modulating endothelial nitric oxide in vascular endothelium. In our study, non-obstructive CAD was seen in 24% of cases.
Low SHBG in postmenopausal women is a potential risk marker for cardiovascular and metabolic morbidity.[17] Low SHBG has been described as an independent predictor of incident type 2 diabetes,[18] reflecting a pre-diabetic state along with obesity, higher glucose, and insulin levels.[19-21] In our study, the ratio of SHBG with HbA1c was analyzed. There was no significant difference in the study and control group.
In postmenopausal women, extremely high concentrations of endogenous testosterone was associated with a high risk of ischemic heart disease and death and extremely low concentrations of endogenous estradiol was associated with a high risk of ischemic heart disease.[22, 23] Our study involved testing endogenous sex hormones only at baseline. There was no difference in the level of estradiol and testosterone between cases and controls. There, however, was an inverse relationship of testosterone to estradiol ratio in cases.
The menopause transitional period is associated with considerable increase in risk for CAD.[23, 24] Studies have shown that distinct alterations occur during menopause transition in endogenous sex hormones, adverse changes in body fat distribution, lipids, and lipoproteins and vascular health. About 44% of our patients were in the 41–50 years age subgroup, the menopausal transition group.
In the Framingham heart study, prolactin levels were not associated with incident CVD risk factors.[25] Higher levels of serum prolactin were seen in cases in comparison to controls in our study. The relevance of it is difficult to interpret in view of small sample size.
CONCLUSION
There was no relationship observed between endogenous sex hormones and acute coronary event in premenopausal women. In addition, whether plasma concentrations of sex steroids reflect the entire hormonal metabolism at the tissue level remains unclear.
Limitations of the study
The study involved small sample size. Serial testing of endogenous sex hormones was not done. The study subjects had only baseline testing.
Acknowledgment
The authors acknowledge the help of Dr Anesh Behl, Endocrinologist, Apollo BGS hospital during the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Audio summary available at
https://doi.org/10.25259/mm_ijcdw_485
References
- Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation. 2019;139:e56-528.
- [Google Scholar]
- Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: A systematic review. Endocrinol Metab Clin North Am. 2013;42:227-53.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: The multi-ethnic study of atherosclerosis. Atherosclerosis. 2012;224:228-34.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multi-ethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289-95.
- [CrossRef] [PubMed] [Google Scholar]
- Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35:471-7.
- [CrossRef] [PubMed] [Google Scholar]
- Does blood pressure differ between users and non-users of hormone replacement therapy? The women's health in the Lund area (WHILA) study. Blood Press. 2002;11:240-3.
- [CrossRef] [PubMed] [Google Scholar]
- Postmenopausal hormone therapy and risk of stroke: The heart and estrogen-progestin replacement study (HERS) Circulation. 2001;103:638-42.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in the regulation of vascular tone. Clin Exp Pharmacol Physiol. 2003;30:1-15.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation. 1996;94:1402-7.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma prolactin is higher in major depressive disorder and females, and associated with anxiety, hostility, somatization, psychotic symptoms and heart rate. Compr Psychoneuroendocrinol. 2021;6:100049.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular disease in women, is it different to men? The role of sex hormones. Climacteric. 2017;20:125-8.
- [CrossRef] [PubMed] [Google Scholar]
- Is there a sex gap in surviving an acute coronary syndrome or subsequent development of heart failure? Circulation. 2020;142:2231-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular disease in women: Clinical perspectives. Circ Res. 2016;118:1273-93.
- [CrossRef] [PubMed] [Google Scholar]
- Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734-44.
- [CrossRef] [PubMed] [Google Scholar]
- Endogenous androgens and sex hormone-binding globulin in women and risk of metabolic syndrome and Type 2 diabetes. J Clin Endocrinol Metab. 2015;100:4595-603.
- [CrossRef] [PubMed] [Google Scholar]
- Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of Type 2 diabetes in men. Eur J Endocrinol. 2010;162:747-54.
- [CrossRef] [PubMed] [Google Scholar]
- Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men, MRFIT research group, Multiple risk factor intervention trial. Am J Epidemiol. 1996;143:889-97.
- [CrossRef] [PubMed] [Google Scholar]
- The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127-35.
- [CrossRef] [PubMed] [Google Scholar]
- Sex hormone-binding globulin and risk of Type 2 diabetes in women and men. N Engl J Med. 2009;361:1152-63.
- [CrossRef] [PubMed] [Google Scholar]
- Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35:471-7.
- [CrossRef] [PubMed] [Google Scholar]
- Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71:2555-66.
- [CrossRef] [PubMed] [Google Scholar]
- Menopause and risk of cardiovascular disease: The Framingham study. Ann Intern Med. 1976;85:447-52.
- [CrossRef] [PubMed] [Google Scholar]
- Association between prolactin and incidence of cardiovascular risk factors in the Framingham heart study. J Am Heart Assoc. 2016;5:e002640.
- [CrossRef] [PubMed] [Google Scholar]







