Translate this page into:
Prognostic Impact of Iron Metabolism Changes in Patients with Acute Coronary Syndrome and its Correlation with TIMI Risk Score and 6-month Left Ventricular Performance
*Corresponding author: Moumita Banerjee, Department of Cardiology, Srirama Chandra Bhanja Medical College Hospital, Cuttack, Odisha, India. moumita_kol1@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Mohanty NK, Dash BK, Satpathy C, Banerjee M, Routray S. Prognostic Impact of Iron Metabolism Changes in Patients with Acute Coronary Syndrome and Its Correlation with TIMI Risk Score and 6-month Left Ventricular Performance. Indian J Cardiovasc Dis Women. doi: 10.25259/IJCDW_61_2023
Abstract
Objectives:
Iron is vital for human metabolism and function. One major cause of anemia is a shortage of iron. On the other hand, a low blood iron level does not always indica te an abnormal hemoglobin concentration. Left ventricular function, ferritin, and blood iron levels were investigated 6 months following the acute coronary syndrome (ACS) index event. In addition, we searched for correlations between thrombolysis in myocardial infarction (TIMI) risk score, cytokines such as C-reactive protein (CRP), and serum iron content.
Materials and Methods:
From August 2021 to July 2022, 100 consecutive patients with ACS who were requesting admission to the critical care unit of the Cardiology Department at SCB Medical College in Cuttack, Odisha, were the subject of this study. Participants in our research with ST-elevation or non-ST-elevation myocardial infarction (ST elevation myocardial infarction [STEMI] or Non ST elevation myocardial infarction [NSTEMI]) ranged in age from 18 to 70 years. We measured CRP, transferrin saturation, total iron binding capacity, ferritin, and blood iron at baseline. The left ventricular ejection fraction (LVEF %) difference was assessed 6 months after baseline echocardiography and follow-up. Various factors were considered while calculating the short- and long-term prognoses, including the patient’s heart failure at admission and any deaths that took place in hospitals within 6 months after the index event.
Results:
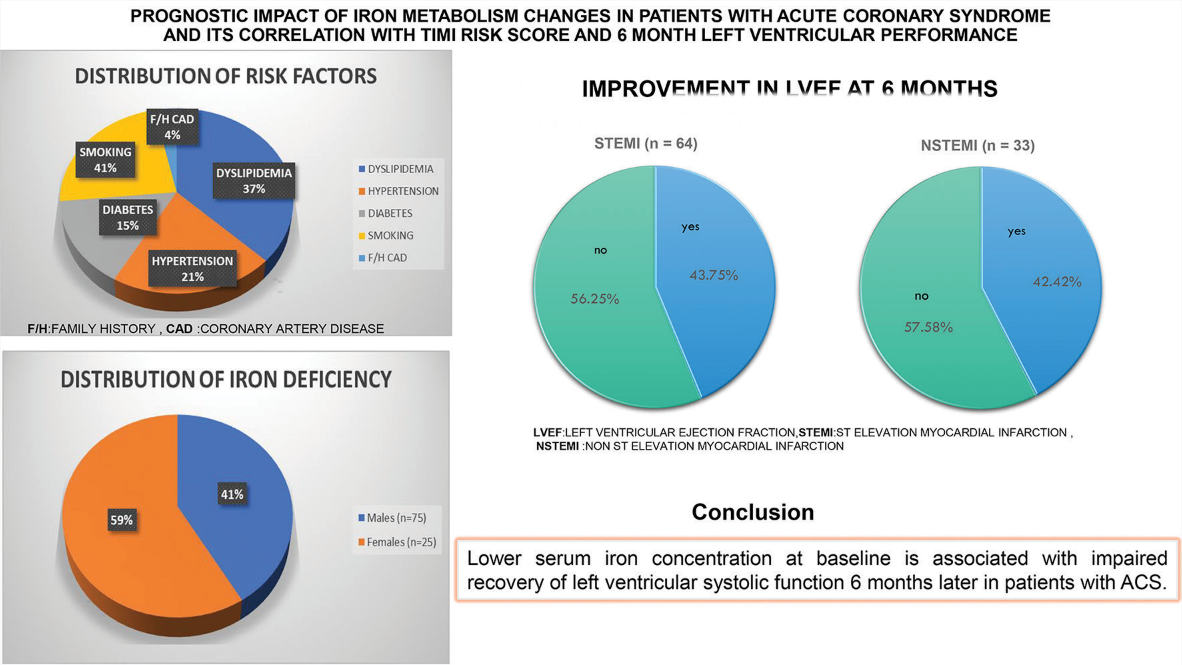
There was no improvement in LVEF for 56.25% of patients (36 out of 66) 6 months after the index event; however, for 43.75% (28 patients) of the 66 STEMI patients, LVEF improved. Out of 34 NSTEMI patients, 14 (42.42%) improved their LVEF 6 months after ACS, whereas 19 (57.58%) did not. Serum iron levels and LVEF at 6 months were shown to be significantly correlated in all patients (overall P < 0.001, STEMI P < 0.001, and NSTEMI P = 0.001). Serum iron levels are positively correlated with improvements in LVEF after 6 months in both patients with STEMI; P < 0.001, and NSTEMI P = 0.006. Serum iron levels and hospitalized STEMI patients’ TIMI risk scores showed a significant connection (P < 0.001).
Conclusion:
Regardless of hemoglobin content, patients with <10% improvement in LVEF from baseline had considerably lower blood iron levels. Six months after their initial ACS incident, patients with ACS who had lower blood iron concentrations at baseline recovered less well in terms of left ventricular systolic function. In our study, 48% of men and 68% of women were found to be iron deficient. In addition to being an indicator of inflammation, hypoferremia may be the target of a novel biomarker with potential applications in medicine in the near future. It could be useful in predicting left ventricular function following ACS.
Keywords
Acute coronary syndrome
ST-elevation myocardial infarction
Non-ST-elevation myocardial infarction
Serum iron ferritin
ABSTRACT IMAGE
INTRODUCTION
Several physiological processes rely on iron, which the human body provides. A component of reticulocytes and erythroblasts, it contributes to the process of erythropoiesis.[1] Storage and oxygen transport are two processes in which iron is involved. Anemia is mostly caused by iron deficiency. Hemoglobin levels, on the other hand, could stay within normal bounds when there is an iron deficiency. Anemia following an acute myocardial infarction (AMI) is linked to a worse prognosis, according to several studies.[2-4] However, little research has looked at how early blood iron levels affect the function of the left ventricle in individuals suffering from acute coronary syndrome (ACS).
Despite iron’s reputation as proatherogenic, studies have shown that low blood iron levels are predictive of a worse outcome in ACS.[5]
Atherosclerotic disease and serum iron levels are both mysteries, and whether or not iron plays a role in ACS is up for debate. Left ventricular function, ferritin, and blood iron levels were investigated 6 months following the ACS index event. In addition, we searched for correlations between TIMI risk score, cytokines such as C-reactive protein (CRP), and serum iron content.
MATERIALS AND METHODS
Subjects and study protocol
One hundred consecutive patients with ACS seeking admission to SCB Medical College’s cardiology department’s critical care unit in Cuttack, Odisha, were studied from August 2021 to July 2022. Study participants included those with ST-elevated myocardial infarction (STEMI) and those with non-ST-elevated myocardial infarction (NSTEMI). The research was carried out using a prospective observational design.
Inclusion criteria
Patients in the 18–70 years age group
ACS patients consist of STEMI and NSTEMI.
Exclusion criteria
AMI patients without baseline iron profile after admission for AMI
Patients who had previous episodes of AMI
Prior history of percutaneous coronary interventions (PCIs) or coronary artery bypass grafting
Past history of inflammatory bowel disease or chronic kidney disease.
Data were collected from the research population’s electrocardiograms, blood tests, and clinical records, including demographic information (gender and age), ACS type, left ventricular ejection fraction (LVEF), personal history, risk factors, and laboratory findings. Venous blood samples were taken at admission after ACS for the purpose of measuring serum iron, transferrin saturation (TSAT), total iron-binding capacity (TIBC), ferritin, and CRP. The laboratory found that typical reference values for serum iron, serum ferritin, TSAT, and total iron binding capacity (TIBC )were 50–170 mcg/dL, 13–400 ng/mL, 15–50%, and 250–425 mcg/dL, respectively.
There were individual patient-specific factors that went into calculating the TIMI risk score for STEMI and NSTEMI. This is how the categorization procedure was executed: In STEMI, groups were divided into a score of one – group 1, score of three – group 2, score of four – group 3, score of five, or more – group 4. The three groups in the NSTEMI study were: Risk score of two as group 1, risk score of three as group 2, risk score of four as group 3.
A GE Healthcare Vivid T8 echocardiogram was performed at the start or 6 months of the trial. Modified Simpson’s approach estimated LVEF. Subtracting the baseline ejection fraction from the 6-month ejection fraction and dividing by the baseline fraction yielded the LVEF% change. The clinical investigation by Ndrepepa et al. found that LVEF changes of 10% or greater suggested cardiac function improvements.[6]
With the patient’s permission, coronary angiography was performed in the cath laboratory using the Siemens Artis Zee Floor model equipment. The angiography results informed the choice to revascularize the patient using catheter angioplasty o r percutaneous coronary intervention (PCI).
In-hospital and 6-month mortality, heart failure, and heart failure at follow-up were the major endpoints.
Statistical analysis
After examining the data in Excel, SPSS (IBM Corp., Released 2020) was used to evaluate it. IBM Corp. in Armonk, New York, released IBM SPSS Statistics for Windows 27.0. For each continuous variable, the mean and standard deviation were calculated. For each category, proportions and frequencies were calculated. To compare continuous variables between study groups, the independent t-test was used. We used Pearson’s correlation coefficient to evaluate continuous variable associations. The Jonckheere-Terpstra test is a rank-based nonparametric test that can be used to determine if there is a statistically significant trend between an ordinal independent variable and a continuous or ordinal dependent variable.
RESULTS
Over the course of the study, we analyzed data from 100 individuals. There were 34% cases of non-STEMI (n = 34) and 66% cases of STEMI (n = 66). Out of the 66 individuals who experienced STEMI, 47 had it in the anterior wall, 15 in the inferior wall, and four in the lateral wall. Of the participants in our research, 75 were men and 25 were female. A family history of coronary artery disease (CAD) was found in 6% of patients, hypertension in 37%, smoking in 27%, diabetes mellitus in 27%, and dyslipidemia in 90% of cases. Of the 66 patients diagnosed with STEMI, 13 cases (19.7%) occurred within 12 h, whereas 53 cases (80.3%) occurred after that period. 12.1% (n = 8) of the ACS STEMI group had a TIMI risk score of 2, 24.25% (n = 16) had each scores of 3 and 4, 16.7% (n = 11) had scores of 5 and 6 each, 3.0% (n = 2) had scores of 7 and 8 each. The percentages of patients with 2, 3, and 4 NSTEMI scores were 41.2% (n = 14), 47.0% (n = 16), and 11.8% (n = 4), respectively. The various biochemical parameters and LVEF at baseline and after 6 months were compared between STEMI and NSTEMI [Table 1].
| Variable | STEMI Mean±SD |
NSTEMI Mean±SD |
Mean Difference | t | P | 95%CI |
|---|---|---|---|---|---|---|
| Age | 54.76±9.51 | 54.82±9.94 | −0.07 | −0.03 | 0.9 | −4.11–3.98 |
| Hb | 12.91±2.02 | 12.51±1.97 | 0.39 | 0.94 | 0.35 | −0. 44–1.23 |
| TLC | 13375.15±3846.62 | 12429.71±3743.34 | 945.45 | 1.17 | 0.24 | −651.55–2542.44 |
| Platelets | 2.67±0.78 | 2.46±0.89 | 0.21 | 1.2 | 0.23 | −0.14–0.55 |
| FBS | 128.47±63.62 | 112.41±28.17 | 16.06 | 1.4 | 0.16 | −6.7–38.82 |
| PPBS | 172.62±97.79 | 149.65±60.04 | 22.97 | 1.25 | 0.21 | −13.44–59.39 |
| Urea | 30.14±12.91 | 27.59±8.84 | 2.55 | 1.03 | 0.3 | −2.35–7.45 |
| Creatinine | 0.86±0.28 | 0.85±0.18 | 0.002 | 0.03 | 0.97 | −0.103–0.106 |
| Cholesterol | 210.36±49.61 | 208.29±50.51 | 2.07 | 0.19 | 0.84 | −18.84–22.98 |
| HDL | 46.86±12.68 | 44.71±12.41 | 2.16 | 0.81 | 0.4 | −3.12–7.43 |
| TG | 136.44±66.23 | 159.74±58.07 | −23.29 | −1.73 | 0.08 | −49.94–3.34 |
| LDL | 132.06±45.11 | 129.79±50.5 | 2.27 | 0.22 | 0.8 | −17.66–22.2 |
| CRP | 36.48±35.11 | 25.68±27.07 | 10.79 | 1.56 | 0.09 | −2.87–24.46 |
| TROP T | 2.36±1.68 | 2.39±2.36 | −0.03 | −0.06 | 0.9 | −0.83—0.78 |
| S Iron | 53.34±25.47 | 63.36±30.52 | −10.02 | −1.74 | 0.08 | −21.45–1.41 |
| Transferrin | 15.09±7.14 | 18.52±9.24 | −3.42 | −2.05 | 0.04 | −6.73–−0.11 |
| TIBC | 365.95±76.25 | 352.47±106.26 | 13.48 | 0.73 | 0.4 | −23.18–50.15 |
| Ferritin | 204.36±171.72 | 236.65±217.2 | −32.28 | −0.73 | 0.46 | −119.76–55.19 |
| LVEF 0 | 44.19±8.75 | 53.58. ±6.62 | −9.39 | −5.41 | <0.001 | −12.83–−5.94 |
| LVEF 6 | 46.73±10.28 | 55.82±9.12 | −9.09 | −4.28 | <0.001 | −13.29–−4.87 |
STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, Hb: Hemoglobin, TLC: Total leukocyte count, FBS: Fasting blood sugar, PPBS: Post-prandial blood sugar, HDL: High-density lipoprotein, TG: Triglyceride, LDL: Low-density lipoprotein, CRP: C-reactive protein, TROP T: Troponin T, S Iron: Serum iron, TIBC: Total iron-binding capacity, LVEF: Left ventricular ejection fraction, SD: Standard deviation, CI: Confidence interval, t: test value, P: P value, LVEF 0:Left ventricular function at baseline (0 month), LVEF(6):Left ventricular function at 6 months
The study comprised the subsequent patient percentages: 45% had single vessel disease, 32% had double vessel disease, 13% had triple vessel disease, 2% had multivessel disease, 3% had non-significant CAD, 1% had normal coronaries, and 4% did not agree for coronary angiography.
Participants’ ferritin and transferrin levels did not change significantly between reference groups, although the frequency of participants’ blood iron levels did (P = 0.011) [Table 2a].
| Variable | Category | STEMI | NSTEMI | Chi-square | P | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Serum iron | Low | 39 | 59.1 | 14 | 41.2 | 6.45 | 0.011 | |
| Normal | 27 | 40.9 | 20 | 58.8 | ||||
| Transferrin | Low | 36 | 54.5 | 16 | 47.1 | 1.11 | 0.29 | |
| Normal | 30 | 45.5 | 18 | 52.9 | ||||
| Ferritin | Low | 2 | 3.0 | 0 | 0.0 | - | - | |
| Normal | 57 | 86.4 | 31 | 91.17 | ||||
| High | 7 | 10.6 | 3 | 8.83 | ||||
| TIBC | Low | 4 | 6.1 | 6 | 17.6 | 7.61 | 0.006 | |
| Normal | 47 | 71.2 | 23 | 67.6 | ||||
| High | 15 | 22.7 | 5 | 14.7 | ||||
STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, TIBC: Total iron-binding capacity, P: P value
In our study, females had a higher prevalence of iron insufficiency (68%) than males (48%) [Table 2b].
| Number of patients | % of patients (as per sex) |
|
|---|---|---|
| Males (n=75) | 36 | 48 |
| Females (n=25) | 17 | 68 |
There was no improvement in LVEF for 56.25 percent of patients (36out of 66 ) six months after the index event,however for 43.75 percent (28 patients) of the 66 STEMI patients,LVEF improved.Out of 34 NSTEMI patients,14(42.42%) improved their LVEF six months after ACS,while 19(57.58%) did not [Table 3].
| STEMI (n=64) | Yes | 28 | 43.75% |
| No | 36 | 56.25% | |
| NSTEMI (n=33) | Yes | 14 | 42.42% |
| No | 19 | 57.58% |
LVEF: Left ventricular ejection fraction, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction
Serum iron and serum hemoglobin have a positive statistically significant connection [Table 4].
| Variable | Group | Mean | SD | r | P |
|---|---|---|---|---|---|
| Hb | Overall | 12.77 | 2.01 | 0.37 | <0.001 |
| Serum Iron | 56.75 | 27.55 | |||
| Hb | STEMI | 12.91 | 2.02 | 0.34 | 0.005 |
| Serum Iron | 53.34 | 25.47 | |||
| Hb | NSTEMI | 12.52 | 1.97 | 0.48 | 0.003 |
| Serum Iron | 63.36 | 30.52 |
r: Pearson ‘s correlation coefficient. Hb: Hemoglobin, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, SD: Standard deviation, P: P value
At 6 months, there is a statistically significant connection between LVEF and serum iron [Table 5].
| Variable | Group | Mean | SD | r | P |
|---|---|---|---|---|---|
| LVEF (6) | Overall | 49.82 | 10.76 | 0.54 | <0.001 |
| Serum Iron | 56.45 | 27.2 | |||
| LVEF (6) | STEMI | 46.73 | 10.28 | 0.53 | <0.001 |
| Serum Iron | 53.68 | 25.68 | |||
| LVEF (6) | NSTEMI | 55.82 | 9.12 | 0.53 | 0.001 |
| Serum Iron | 61.82 | 29.62 |
LVEF: Left ventricular ejection fraction, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, SD: Standard deviation, LVEF(6): Left ventricular function at 6 months, P: P value, r: Pearson ‘s correlation coefficient
Statistical analysis reveals a correlation between elevated serum iron levels and better LVEF after 6 months [Table 6].
| Variable | Group | Test statistics | P |
|---|---|---|---|
| Serum Iron | Overall | 5.34 | <0.001 |
| STEMI | 4.66 | <0.001 | |
| NSTEMI | 2.77 | 0.006 | |
| Ferritin | Overall | 0.58 | 0.56 |
| STEMI | 0.78 | 0.43 | |
| NSTEMI | 0.09 | 0.93 |
Jonckheere–Terpstra test, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, EF: Ejection fraction, P: P value
There was a statistically significant correlation (P < 0.001) between serum iron and TIMI risk score at admission in STEMI patients [Table 7].
| Variable | Group | Test statistics | P |
|---|---|---|---|
| Serum Iron | STEMI | 4.54 | <0.001 |
| NSTEMI | −0.51 | 0.6 | |
| Ferritin | STEMI | −0.52 | 0.6 |
| NSTEMI | 0.45 | 0.6 |
Jonckheere–Terpstra test, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-ST-elevation myocardial infarction, P: P value, TIMI: Thrombolysis in myocardial infarction
At the time of admission, there was no statistically significant correlation between serum ferritin and TIMI risk score (P = 0.6), serum ferritin and improvement in LVEF at 6 months (P = 0.56), serum ferritin and CRP (P = 0.08), serum ferritin and LVEF at baseline (P = 0.28), and serum ferritin and improvement in LVEF at 6 months (P = 0.56).
The Jonckheere-Terpstra test used in Tables 6 and 7 is used to determine if there are statistically significant differences between two or more groups of an independent variable (serum iron and ferritin) on a dependent variable (improvement in EF in table 6 and TIMI score in table 7).
DISCUSSION
The study indicated that the mean age at presentation was 54.78 ± 9.61 years and that there were 75 male participants and 25 female participants, with 75% of the total being male and 25% being female. Duarte et al.[7] found that 45% (n = 125) of 280 consecutive patients with STEMI and 44% (n = 122) with NSTEMI. A total of 204 out of 280 patients, or 73% of the total were male, and their average age at presentation was 68 ± 13 years.
Ninety (or 90%) of the 100 individuals who participated in our study reported dyslipidemia as their main concern. Duarte et al.[7] found that half of the patients (140 people) had dyslipidemia and 32% had diabetes.
After 6 months, 28 out of 66 STEMI patients (43.75%) had improved LVEF, whereas 36 (56.25%) patients exhibited no change. For their study, Huang et al.[8] looked at 55 individuals in a row who had a de novo acute STEMI. A total of 36 (65%) patients showed no change in EF over 10%, whereas 19 (35%) patients had an improvement in EF > 10%.
There are statistically significant positive relationships between serum iron and many variables at 6 months, including LVEF improvement, hemoglobin, and serum iron and hemoglobin.
Serum iron concentration and hemoglobin concentration, as well as the concentration of LVEF at 6 months, showed a positive correlation (Rho correlation coefficient: 0.273, P = 0.036), according to research by Huang et al.[8] With a Rho correlation coefficient of 0.301 and a P = 0.025, the baseline blood iron level was considerably lower in patients who did not demonstrate any improvement in left ventricular function at the 6-month follow-up. The group that did not experience any improvement had a significantly lower serum iron concentration (80.8 ± 50.8 vs. 52.7±24.1 µg/dL, P = 0.016) compared to the group that saw improvement under the same condition.
At the time of admission, there is a substantial correlation (P < 0.001) between the serum iron level and the TIMI risk score for patients who have sustained STEMI. On the other hand, there is no such link (P = 0.6) for patients who suffered a NSTEMI. Within 6 months after admission, one patient (1% of the total) died from heart failure while hospitalized. Two further patients died (cardiac reasons) within 6 months of follow-up. Three individuals, or 3.0% of the total, died during our research.
CONCLUSION
Patients whose LVEF improved by <10% from baseline had significantly reduced blood iron levels, regardless of hemoglobin content. Patients with ACS who had lower blood iron concentrations at baseline had a worse recovery in left ventricular systolic function 6 months following their initial ACS event. In our study, the prevalence of iron insufficiency was 48% in men and 68% in women. According to this study, women are more likely than males to be iron deficient. In addition to being an indicator of inflammation, hypoferremia may be the target of a novel biomarker with potential applications in medicine in the near future. It could be useful in predicting left ventricular function following an ACS.
Ethical approval
The research/study was approved by the Institutional Review Board at SCB MEDICAL COLLEGE HOSPITAL, number 993, dated 4.2.2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Audio summary available at
Financial support and sponsorship
Nil.
References
- Prevalence, Incidence, and Prognostic Value of Anaemia in Patients after an Acute Myocardial Infarction: Data from the OPTIMAAL Trial. Eur Heart J. 2009;30:1331-9.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in Haemoglobin Levels during Hospital Course and Long-term Outcome after Acute Myocardial Infarction. Eur Heart J. 2007;28:1289-96.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of Anemia in Patients with Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (Cadillac) Trial. J Am Coll Cardiol. 2004;44:547-53.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of Serum Ferritin in the Acute Coronary Syndrome: A Puzzle Still to be Resolved? Int J Cardiol. 2012;154:215.
- [CrossRef] [PubMed] [Google Scholar]
- Evolution of Left Ventricular Ejection Fraction and Its Relationship to Infarct Size after Acute Myocardial Infarction. J Am Coll Cardiol. 2007;50:149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Impact of Iron Metabolism Changes in Patients with Acute Coronary Syndrome. Arq Bras Cardiol. 2018;111:144-50.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Iron Concentration, but not Hemoglobin, Correlates with TIMI Risk Score and 6-month Left Ventricular Performance after Primary Angioplasty for Acute Myocardial Infarction. PLoS One. 2014;9:e104495.
- [CrossRef] [PubMed] [Google Scholar]








