Translate this page into:
Silent Intruder: Unveiling Asymptomatic Anomalous Left Coronary Artery in Adulthood
*Corresponding author: Mohammad Shibly Ashhar, Department of Respiratory Medicine, Asram Medical College and Hospital, Eluru, Andhra Pradesh, India. mdashhar14@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tammiraju I, Teja DA, Ashhar MS, Kusuma M. Silent Intruder: Unveiling Asymptomatic Anomalous Left Coronary Artery in Adulthood. Indian J Cardiovasc Dis Women. 2024;9:241-5. doi: 10.25259/IJCDW_31_2024
Abstract
Anomalous left coronary artery from the pulmonary artery (ALCAPA), a rare congenital heart anomaly, typically manifests with heart failure symptoms in infants and carries a high mortality rate without surgical intervention. Patients are categorized into adult and infantile types based on collateral presence, impacting cardiovascular risk. Interestingly, some patients may remain asymptomatic for decades due to adequate collateral development, with a decreasing risk of sudden death as they reach adulthood. Management of asymptomatic cases requires individualized consideration and diagnostic confirmation before intervention, despite decreasing risk of sudden death with age. We report a case involving a 35-year-old asymptomatic woman who came for a routine annual check-up. While her electrocardiography, ECHO was normal, a positive Treadmill stress test prompted coronary angiography, revealing an incidental diagnosis of ALCAPA. Subsequently, the patient was referred to cardiothoracic and vascular surgery for further evaluation and necessary interventions, but the patient denied surgery as she did not accept the risk in view of asymptomatic status.
Keywords
anomalous left coronary artery from the pulmonary artery
adult
ECHO
coronary angiography
surgery
INTRODUCTION
Also known also as Bland White Garland syndrome, anomalous left coronary artery from the pulmonary artery (ALCAPA) is an uncommon congenital cardiac defect that is typically diagnosed in early childhood. It is responsible for about 0.25–0.5% of congenital heart disorders and affects 1 in 300,000 newborns.[1] The symptoms usually start to show up at about 3 months of age, and if they are not addressed with surgery, they often result in heart failure and death. Due to collateral blood flow from the right coronary artery (RCA), some people might not have any symptoms. In adulthood, they may experience mild to severe symptoms, such as angina, arrhythmias, heart failure, mitral valve leak, shortness of breath, and restricted exercise tolerance. We report a case of ALCAPA discovered incidentally in an adult female during routine medical assessment, emphasizing that due to effective collateral circulation, the condition may continue without symptoms.
CASE REPORT
A 35-year-old female patient presented for a routine check-up with occasional shortness of breath of New York Heart Association class II. The electrocardiography (ECG) did not exhibit any noteworthy alterations [Figure 1a]. The initial survey did not reveal any major regional wall motion abnormalities and has normal echocardiogram findings, including an ejection fraction of 60% and Grade 1 diastolic dysfunction. However, on detailed study, parasternal short axis (PSAX) view in ECHO showed some subtle features like turbulence originating from pulmonary artery (PA) (shown by arrow) into a small channel [Figure 1b]. We were unable to trace it exactly. With these findings in mind, we performed a coronary angiogram for further evaluation of positive stress test and to investigate ECHO findings.
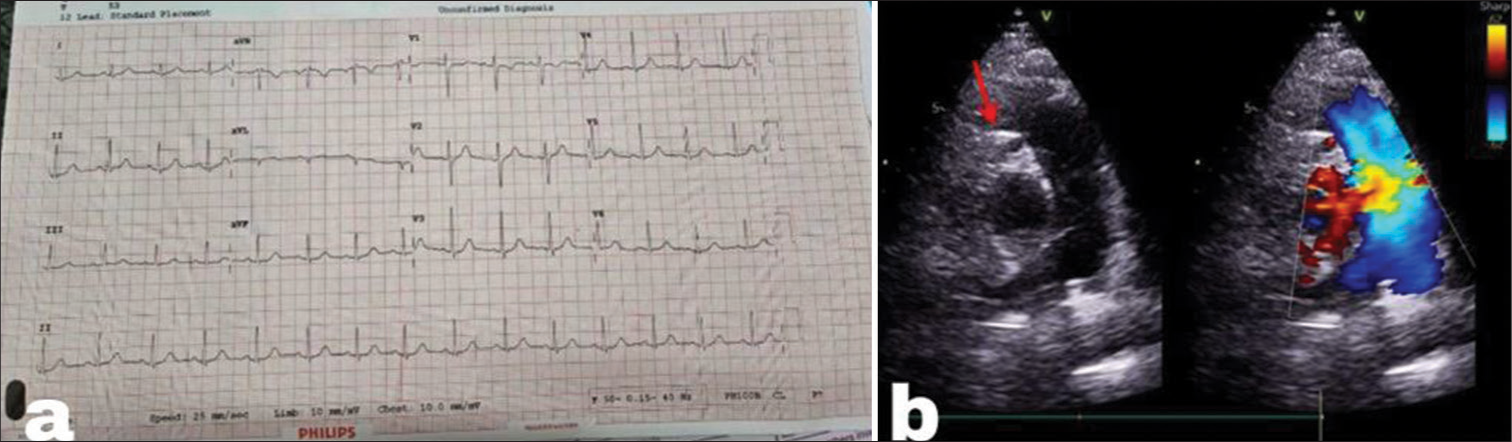
- (a) Electrocardiography with no significant ST-T changes. (b) Parasternal short-axis echocardiogram view depicts a small anomalous channel (red arrow) representing the left coronary artery draining from the pulmonary artery.
During the angiogram, as we were initially unable to engage the left system, we resorted to engaging the right system, revealing retrograde filling of the entire left coronary artery [Figure 2a]. Unexpectedly, there was extravasation of dye into a major artery which was an uncommon finding in cases of tight left main lesions, suggesting a potential unusual origin of the left coronary artery (LCA). This extravasation of dye had given us a crucial clue, suggesting that this site of retrograde filling into the major artery could be in the PA. On further investigation with pigtail injection into the PA, we have confirmed that LCA is arising from PA [Figure 2b], that leads to the diagnosis of ALCAPA [Figure 2c]. Retrogradely, we had confirmed that the channel seen in parasternal short axis view PSAX view coming from PA has a left coronary artery. The patient was subsequently referred to the cardiothoracic and vascular surgery department. However, due to the patient’s asymptomatic status, they declined surgery and are currently under regular follow-up, maintaining a stable condition.
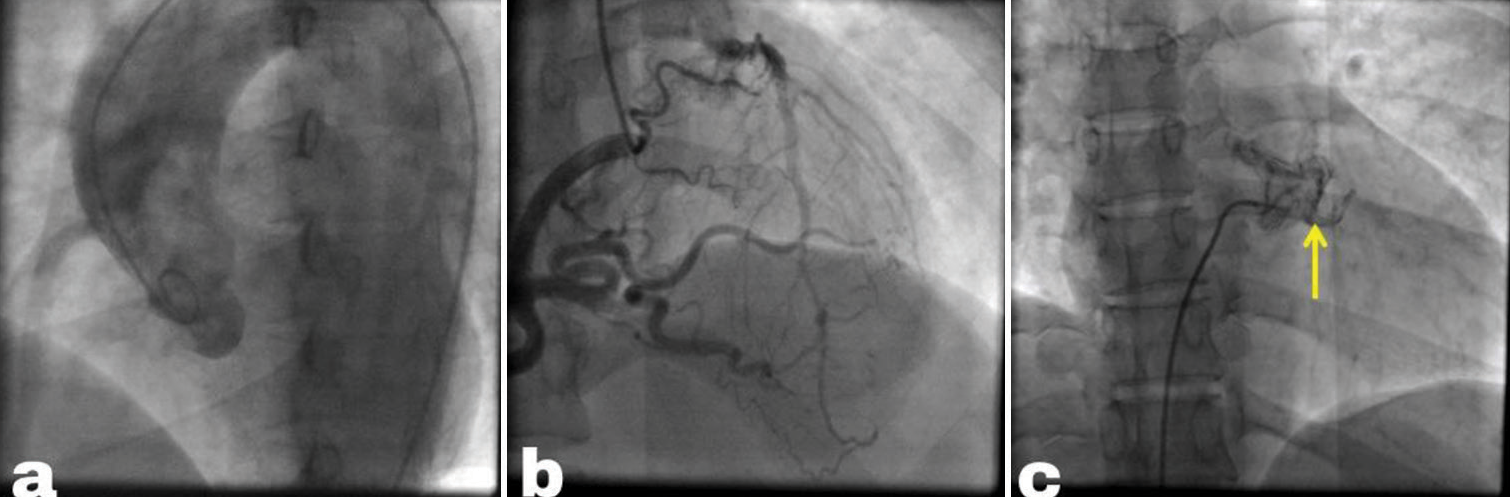
- (a) Pigtail aortogram displays an empty left sinus with no coronary arteries originating from it. (b) Angiogram of the right coronary artery (RCA) reveals a significantly enlarged RCA with retrograde filling of the left coronary artery, draining into the pulmonary artery (PA). (c) PA angiogram showing partial filling of the left coronary artery (yellow arrow) due to competitive flow.
DISCUSSION
The abnormal origin of a coronary artery from PA was initially reported by Brooks in 1882, but a comprehensive clinical description did not appear until 1933.[2] ALCAPA is a rare congenital heart condition, affecting about 1 in 300,000 newborns and constituting 0.25–0.50% of cases of congenital heart disease.[1] About 90% of patients die from myocardial ischemia and heart failure during the 1st year if they are left untreated. Only about 18–25% of patients with this condition reach adulthood.[3]
Abnormal placement of the coronary buds or abnormal rotation of the spiral septum that divides the truncus is the two embryological causes of coronary abnormalities.[4] Pulmonary circulation changes following birth are the cause of the syndrome’s clinical presentation. The balance between ductus closure and collateral development determines outcomes. There was no need for collateral vessel formation before birth as the patent ductus arteriosus maintains equal pressures in the main PA and aorta, ensuring the normal myocardial perfusion. However, immediately after birth, high pulmonary circulation resistance allows flow from PA to the left coronary artery (LCA), perfusing the left ventricle and reducing the likelihood of symptoms in neonates. A few weeks to months after birth, as the ductus arteriosus shuts and pulmonary vascular resistance decreases, blood supply to the LCA ceases, relying on collaterals from RCA, with survival dependent on collateral development. Reduced PA pressure causes reversal of blood flow from LCA to the PA, leading to the steal phenomenon.[5] This coronary steal can cause mitral insufficiency due to ischemia that affects the ventricular walls and papillary muscles, as well as insufficient oxygenation and overt ischemia in the left myocardial tissue, which can result in the left ventricular (LV) diastolic overload.
Due to restricted or missing coronary collateral flow, infants with ALCAPA frequently experience early myocardial ischemia, LV dysfunction, dilatation, and mitral regurgitation. They also frequently exhibit pallor, poor weight gain, rapid breathing, and an increased heart rate. When it comes to adults, the average age of presentation is 40.6 years.[6] Continuous murmurs are frequently heard from collaterals, but they can also be exclusively systolic when pulmonary arterial hypertension persists. Rhythm disorders in adulthood caused by changes in the cardiac electrical conduction system may hide underlying myocardial ischemia and result in sudden cardiac death. Some people with ALCAPA may have normal LV systolic function but still have dyspnea due to diastolic dysfunction, increased LV end-diastolic pressure. Anomalies of the coronary artery can be life-threatening during or after severe exertion. The risk of sudden death appears to decrease after age 50. Our patient escaped the infantile stage probably due to adequate collaterals from RCA supplying LCA.
Diagnosis
Clinical features, ECG, echocardiography, and imaging are used to make the diagnosis. Both at rest and during activity testing, the ECG may exhibit ischemia symptoms, such as significant ST segment elevations and Q waves, particularly in the anterior and lateral leads.[7] Our patients ECG is normal without any significant ST segment elevations. In echocardiography, parasternal aortic root short-axis view shows typical findings in ALCAPA including the left coronary artery (LCA) arising from PA rather than the aorta. Shunting from RCA to the major PA through the left collateral artery (LCA) is indicated by retrograde blood flow in collaterals. In addition, the proximal RCA may appear dilated and tortuous. ECHO also reveals anomalies in regional wall motion, LV dilatation, and mitral regurgitation, which may be functional (as a consequence of LV dilatation) or associated with fibrosis and ischemia of the papillary muscles.[8] Our patient’s ECHO revealed an aberrant artery originating from the pulmonary trunk, establishing a probable diagnosis of ALCAPA, along with normal chambers and grade I diastolic dysfunction with adequate systolic function.
Computed tomography angiogram is usually preferred as it has superior spatial and temporal resolution. We performed conventional coronary angiography in our case, which further demonstrated that there is no coronary artery that has left sinus origin. It also showed that the RCA is notably enlarged, with multiple collaterals supplying the left coronary artery (LCA) and draining into PA. This angiographic finding confirms the diagnosis of ALCAPA, as the RCA is retrogradely draining through the LCA into PA.
Treatment
In adults, due to the size of the heart and compensatory issues related to the coronary circulation to the left ventricle, surgical repair is difficult. The two main treatments for ALCAPA are ligating the abnormal left coronary artery and creating a dual coronary artery system.[9] This can be done through various methods, such as direct connection to the aorta, vein graft, conduits from the carotid or subclavian artery, or the Takeuchi procedure. Therefore, in older patients aged 50 and above, conservative approach may be considered due to the increased risks associated with surgery.[10] Similarly, our patient, though in her third decade, opted against surgery due to her asymptomatic status and the high surgical morbidity. The salient features of ALCAPA syndrome are mentioned in Table 1.
| 1. INCIDENCE |
| 1/300,000 newborns |
| 18–25% reach adulthood |
| 2. EMBRYOGENESIS |
| Abnormal rotation of the spiral septum that divides the truncus or |
| Abnormal positioning of the coronary buds |
| 3. PATHOPHYSIOLOGY-Depends on ductus closure and collateral development |
| Before birth–Pulmonary artery pressure=Aorta pressure. So, No Collateral vessel formation |
| Immediately after birth-high pulmonary circulation resistance. PA→LCA flow. Still no symptoms |
| Weeks to months after birth |
| PA Pressure drops. Reversal of flow LCA→PA flow. (Coronary steel phenomenon occurs and symptoms due to ischemia) |
| Collaterals develop from RCA to LCA |
| 4. CLINICAL FEATURES |
| IN INFANTS |
| Myocardial ischemia, |
| Left ventricular dysfunction, and |
| Mitral regurgitation (due to limited coronary collateral flow) |
| Symptoms-pallor, poor weight gain, rapid breathing, and fast heart rate |
| IN ADULTS |
| Mostly Asymptomatic |
| Shortness of breath/limited exercise tolerance/heart failure/mitral valve leakage/angina/arrhythmias |
| May present with continuous murmurs [indicating collateral circulation] |
| Might lead to sudden cardiac death |
| 5. DIAGNOSIS |
| ECG |
| Ischemic signs, including significant ST segment elevations and Q waves, particularly in the anterior and lateral leads (May be seen both at rest and during exercise testing) |
| ECHOCARDIOGRAPHY |
| LCA originates from PA (parasternal aortic root short-axis view) |
| Retrograde blood flow in collaterals from RCA to LCA. |
| Proximal RCA appears dilated and tortuous. |
| Dilatation of LV, regional wall motion abnormalities, mitral regurgitation (functional/papillary muscle ischemia/fibrosis) |
| CT ANGIOGRAM |
| Has superior spatial and temporal resolution. |
| Confirms absence of LCA originating from the left sinus. |
| Significantly enlarged RCA with multiple collaterals supplying LCA, draining into the pulmonary artery |
| 6. TREATMENT |
| Ligation of abnormal LCA and creation of dual coronary artery system |
| Methods |
| Coronary button transfer [preferred method in infants] |
| Direct connection with vein graft to establish LCA to Aorta continuity |
| Conduits from carotid or subclavian artery to the anamolous coronary artery |
| Takeuchi procedure [transpulmonary baffling] |
| Increased morbidity due to surgery (21%) |
| Conservative management for patients >50 years |
PA: Pulmonary artery, RCA: Right coronary artery, LV: Left ventricle, ALCAPA: Anomalous left coronary artery from the pulmonary artery, LCA: Left coronary artery
CONCLUSION
ALCAPA, a rare congenital heart condition, that often leads to high infant mortality and rarely shows symptoms in adults. Our case report demonstrates how ALCAPA can go years without exhibiting any symptoms. Even though the initial risk of sudden death decreases with age, the management of asymptomatic patients remains debatable, requiring specific individualized considerations and diagnostic confirmation before intervention.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Anomalous Origin of Left Coronary Artery from Pulmonary Artery (ALCAPA): A Case Report. Radiol Case Rep. 2022;17:3432-5.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital Anomalies of the Coronary Arteries: Report of an Unusual Case Associated with Cardiac Hypertrophy. Am Heart J. 1933;8:787-801.
- [CrossRef] [Google Scholar]
- Case Report: ALCAPA Syndrome: Successful Repair with an Anatomical and Physiological Alternative Surgical Technique. F1000Res. 2016;5:1680.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical Repair for Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery. Korean Circ J. 2017;47:144-7.
- [CrossRef] [PubMed] [Google Scholar]
- Adult-type ALCAPA Syndrome: A Rare Coronary Artery Anomaly. Echocardiography. 2018;35:1056-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sudden Cardiac Arrest in an Adult with Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery (ALCAPA): Case Report. Int J Environ Res Public Health. 2022;19:1554.
- [CrossRef] [PubMed] [Google Scholar]
- ALCAPA in Adult Asymptomatic Patient: A Case Report. Int J Surg Case Rep. 2023;109:108521.
- [CrossRef] [PubMed] [Google Scholar]
- Anomalous Origin of the Left Coronary Artery from the Main Pulmonary Artery Detected on Echocardiographic Screening Study of Newborns. Int J Cardiol. 2004;97:561-2.
- [CrossRef] [PubMed] [Google Scholar]
- The Midterm Outcomes of Coronary Artery Bypass Grafting for Adult Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery. Indian J Thorac Cardiovasc Surg. 2024;118:519-20.
- [CrossRef] [Google Scholar]
- Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery in Adults: A Comprehensive Review of 151 Adult Cases and a New Diagnosis in a 53-year-old Woman. Clin Cardiol. 2011;34:204-10.
- [CrossRef] [PubMed] [Google Scholar]








