Translate this page into:
Proteinuria as a Marker for Cardiovascular Disease in Renal (Chronic Kidney Disease) Patients
Meghana Kodali, 2nd year MBBS Gandhi Medical College Sahara Estate, Hyderabad, 500074 India meghanakodali28@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background For the evaluation of total cardiovascular risk in patients with chronic kidney disease (CKD; renal), the amount of protein in the urine has to be considered an important factor. For reducing proteinuria level and lowering the risk of renal, cardiovascular endpoint, this may become a crucial decision.
Materials and Methods This is a case-control study of the stage 5 CKD female patients recruited over 2 months in 2017, based on clinical and laboratory investigation. CKD was diagnosed on serum creatinine levels and stage 5 CKD depending on estimated glomerular filtration rate (eGFR) calculation. Coronary artery disease (CAD) was diagnosed on clinical history, electrocardiogram (ECG), and echocardiogram.
Results This study was conducted on 50 patients at the authors’ hospital. Out of these, 25 were cases and 25 constituted controls. Out of 25 controls, 13 had microalbuminuria and 12 had proteinuria and no cardiovascular disease. Out of 25 cases, 2 cases had microalbuminuria and 23 had proteinuria. More number of CKD with CAD group had proteinuria than CKD without CAD, which was statistically highly significant (p < 0.000). CKD patients with CAD had higher degree of proteinuria than those without CAD, which was statistically significant (p < 0.005).
Conclusion This study showed that proteinuria and its cardiovascular outcomes in CKD patients are correlated. For detection of CAD in CKD patients, proteinuria levels may be crucial regarding the treatment decision.
Keywords
chronic kidney disease
cardiovascular disease
proteinuria
electrocardiograph
Introduction
Patients with chronic kidney disease (CKD) are at high risk for cardiovascular disease (CVD).1 Patients who reach CKD stage 5 and enter dialysis have high rate of cardiovascular death.2 CVD is the leading cause of premature death among the CKD population.3 It is now widely accepted that proteinuria is an independent predictor of cardiovascular morbidity and mortality across divergent populations,4 in addition to its role as a marker for the risk of CKD.
Proteinuria, specific to microalbuminuria, is studied as risk factor for long-term cardiovascular and all-cause mortality in high-risk patients such as diabetes and hypertension.5 6 As proteinuria can be detected by dipstick test, this may be the screening test for this high-risk population as it is cost-effective.7
Other advantage of proteinuria is that it can be detected earlier than the renal function deterioration by the glomerular filtration rate.3 Proteinuria is not only considered as a marker of long-term cardiovascular events, but it can also reflect the control of blood pressure.8 9
However, there are limited studies in female populations, so the authors aimed at this population.
Materials and Methods
This is a case-control study based on clinical and laboratory investigation. This study was carried out in the Department of Biochemistry and Department of Nephrology at the authors’ hospital, during May and June 2017. A total of 50 patients were enrolled in this study, and they were divided into two groups. The first group consists of 25 women with CKD in stage 5 and CAD, and the second group consists of 25 women with CKD in stage 5 and without CAD. Clinical history, electrocardiogram (ECG), and echocardiogram were done to detect the CAD.
Inclusion criteria: Women of age group 25 to 65 years with CKD in stage 5.
Exclusion criteria: Women with chronic liver disease, cerebrovascular disorders, uncontrolled endocrine disorder such as gross hypothyroid patients, active psoriasis, or active tuberculosis. Pregnant, lactating women, and chronic alcoholics are excluded from this study.
From all patients, 3 mL of blood was drawn and centrifuged of which 2 mL of serum was taken for testing serum creatinine level to confirm that they are CKD patients. Creatinine levels of 0.5 to 1.1 mg/dL are considered as normal.
The parameters noted from the ECG include heart rate, PR interval, QRS duration, QRS axis, corrected QT interval, and ST-T changes. PR interval 0.12 to 0.20 second, QRS duration 0.06 to 0.10 second, P-wave dispersion 0.272 ± 0.053, and QRS axis of +90 to −30 degrees are considered as normal (as published by Journal of the American Society of Nephrology 10).
Midstream 3 mL urine sample was taken from all patients, and dipstick proteinuria test was performed. The test method consists of immersing the test strip completely in a well-mixed sample of urine for a short period of time, then extracting it from the container and supporting the edge of the strip over the mouth of the container to remove excess urine. The strip is then left to stand for the time necessary for the reactions to occur (usually 1–2 minutes), and finally the colors that appear are compared against the chromatic scale provided by the manufacturer. The normal value is less than 100 mg/dL.
Statistical Analysis
The findings were analyzed by student t-test to calculate the p value for significance. p value of < 0.05 was considered as significant.
Results
Total 25 cases and 25 controls were studied. Out of 25 cases, 23 (92%) patients were hypertensive, 4 (16%) were diabetic, and 4 (16%) were hypothyroid. Mean age of this group was 45.6 years. All cases were patients with known chronic stable angina by history and coronary angiogram. On ECG, 17 (68%) patients have left ventricular hypertrophy (LVH) and 1 (4%) patient showed small voltage complexes. On echocardiogram, four (16%) patients had pericardial effusion and three (12%) had dilated cardiac chambers with mild left ventricular (LV) dysfunction.
Out of 25 controls, 19 (76%) were hypertensive patients, 12 (48%) were diabetic, and 2 (8%) were hypothyroid patients. Mean age of this group was 43.9 years. ECG and echocardiogram were within normal range.
Serum creatinine levels and eGFR (Table 1) were comparable in both cases and controls.
|
Group |
Mean |
SD |
Maximum |
Minimum |
p Value |
|---|---|---|---|---|---|
|
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation. |
|||||
|
Serum creatinine (mg/dL) |
|||||
|
Cases |
7.06 |
2.9 |
14.1 |
2.3 |
0.84 |
|
Controls |
6.16 |
2 |
10.2 |
2.7 |
|
|
eGFR (mL/min) |
|||||
|
Cases |
3.06 |
1.5 |
1 |
25 |
0.11 |
|
Controls |
4.17 |
3 |
2 |
28 |
|
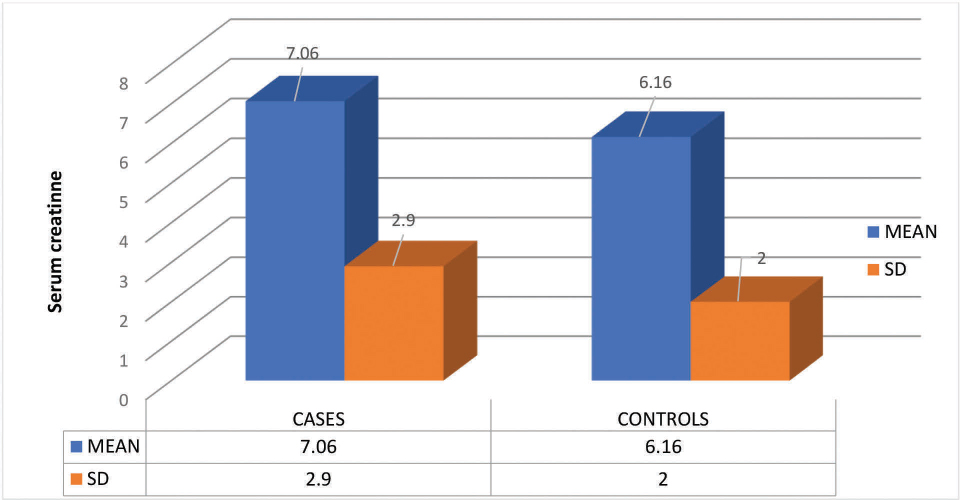
Fig. 1 shows bar diagram of the serum creatinine (mg/dL) levels in this study population.
-
Fig. 1 Bar diagram showing serum creatinine levels in this study population. SD, standard deviation.
Fig. 1 Bar diagram showing serum creatinine levels in this study population. SD, standard deviation.
Table 2 shows that in 25 cases, 23 (92%) patients had proteinuria and 2 (8%) had microalbuminuria, whereas in controls, 12 (48%) had proteinuria and 13 (52%) had microalbuminuria. More cases had proteinuria when compared with controls. More controls had microalbuminuria when compared with cases. Fig. 1 shows the mean and SD values of serum creatinine levels in study population.
|
Urinary protein levels |
Cases |
Controls |
|---|---|---|
|
Microalbuminuria |
2 |
13 |
|
Proteinuria |
23 |
12 |
|
Total |
25 |
25 |
Further, the authors tested the degree of difference of proteinuria in cases and controls (Table 3). Comparing the cases and controls that had proteinuria (Fig. 2), the cases had more degree of proteinuria than the controls, which was also statistically significant (0.005).
|
Group |
Mean (mg/dL) |
SD |
p Value |
|---|---|---|---|
|
Abbreviation: SD, standard deviation. |
|||
|
Controls |
568.8 |
560 |
0.005 |
|
Cases |
1039.6 |
570.6 |
|

-
Fig. 2 Bar diagram showing comparison of degree of proteinuria in both groups. SD, standard deviation.
Fig. 2 Bar diagram showing comparison of degree of proteinuria in both groups. SD, standard deviation.
Discussion
The present study showed that proteinuria has evolved into a surrogate marker of cardiovascular risk, which is supported by multiple studies that have demonstrated that there is an independent association between proteinuria and other surrogate markers of CVD. Urinary protein excretion has not only reflecting the localized subclinical disease but also generalized vascular endothelial dysfunction that was surrogated by steno hypothesis.11
Proteinuria through the nitric oxide inhibition produces endothelial dysfunction. This may be the mechanism for long-term CVDs along with prothrombotic state.12 13
The present study correlates with The Prevention of Renal and Vascular End Stage Disease (PREVEND)14 study. In both studies, albuminuria and cardiovascular risk showed a good correlation. According to the PREVEND study, additionally, when albumin excretion is more than 300 mg, authors showed that this degree of proteinuria increased the risk for sixfold.
In ADVANCE15 (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) study also, albuminuria and cardiovascular risks were well proven, but diabetic patients’ percentage was very high in contrast to the present study that showed only 16% of cases as diabetic. Lower incidence of diabetes mellitus (DM) in this study population may be due to the selection bias than reality.
The present study showed that proteinuria is strongly associated with CAD, which is supported by the U.S. National Health and Nutrition Examination Survey. Egan et al in this study showed correlation of albuminuria and CVD like this study.16
The major limitation of this study is its small sample size. There is lack of follow-up of patients. Another one is that the authors tested the presence and degree of proteinuria in stage 5 CKD patients who were already known cases of CAD, rather than following the CKD patients with proteinuria prospectively for the development of CAD.
In conclusion, more number of CAD with stage 5 CKD patients had proteinuria. Not only just the presence of proteinuria was significantly high in CAD with CKD patients (p = 0.000), but the degree of proteinuria was also significantly high (p = 0.005) in these patients when compared with CKD patients without CAD. Hence early screening for proteinuria in CKD patients may prevent the risk of developing cardiovascular complications and mortality.
Possible interpretation of this study is that even though the authors have significant limitations in their study, proteinuria at any stage of the CKD still represents the long-term marker for CVD.
References
- American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and PreventionCirculation. 2003;108(17):2154-2169.
- [Google Scholar]
- Manchester Institute of Nephrology and Transplantation, Manchester Royal Infirmary, Manchester, UK Correspondence: Alastair Hutchison, Renal Dialysis Unit, The Royal Infirmary, Oxford Road, Manchester M13 9WL, UK.
- [Google Scholar]
- Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2013;7:13-24.
- [Google Scholar]
- Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009;6(04):301-311.
- [Google Scholar]
- Risk Factor Intervention Study Group. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitusAm J Cardiol. 1997;80(02):164-169.
- [Google Scholar]
- Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general populationCirculation. 2002;106(14):1777-1782. Oct 1
- [Google Scholar]
- Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32(12):1493-1499.
- [Google Scholar]
- ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular diseaseJ Am Soc Nephrol. 2011;22(07):1353-1364.
- [Google Scholar]
- Electrocardiographic measures and prediction of cardiovascular and noncardiovascular death in CKD. J Am Soc Nephrol. 2016;27(02):559-569.
- [Google Scholar]
- Albuminuria reflects widespread vascular damage. The steno hypothesisDiabetologia. 1989;32(04):219-226.
- [Google Scholar]
- ADMA levels correlate with proteinuria, secondary amyloidosis, and endothelial dysfunction. J Am Soc Nephrol. 2008;19(02):388-395.
- [Google Scholar]
- Albuminuria is directly associated with increased plasma PAI-1 and factor VII levels in NIDDM patients. Diabetes Res Clin Pract. 1997;36(01):11-18.
- [Google Scholar]
- Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and non-cardiovascular mortality in general populationCirculation. 2002;106(14):1777-1782.
- [Google Scholar]
- ADVANCE Collaborative Group. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetesJ Am Soc Nephrol. 2009;20(08):1813-1821.
- [Google Scholar]
- US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
- [Google Scholar]







