Translate this page into:
Management of COVID-19
Mary John, MD Department of Medicine, Christian Medical College and Hospital, Ludhiana Punjab, 141008 India mjohncmc@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The SARS-CoV-2 (COVID-19) pneumonia was first seen in Wuhan province, China, in December 2019. Since then, there has been great efforts toward understanding the pathophysiology and management of this disease, which was declared as a pandemic in March 2020. Medications which were initially used have been removed and newer ones are under clinical evaluation. Oxygen therapy, anti-inflammatory and antiviral medication, along with awake proning are currently being used in the management of COVID-19.
Keywords
CCC
DCHC
Management of COVID-19
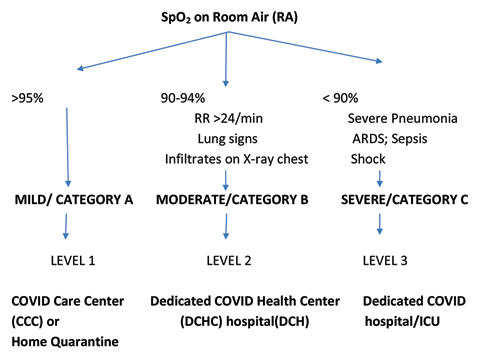
The COVID-19 pandemic has been evolving since December 2019, and so has the understanding of the disease and its management. Siddiqi and Mehra in their paper on “COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal” have eloquently described the various stages of COVID-19 disease (Fig. 1) along with some potential therapies.1 As the pandemic evolved, multiple observational studies were done without proper controls.
-
Fig. 1
Triaging of confirmed COVID-19 patients.
Fig. 1 Triaging of confirmed COVID-19 patients.
Since there are several ongoing studies/clinical trials, a simple treatment protocol could be adopted, based on the original staging of the disease along with the added thrombotic complications (Table 1). These treatments may also be subject to change as and when results of these studies are endorsed by various regulatory bodies (ICMR, IDSA FDA, etc.).
|
Stage I (early infection) |
Stage II (pulmonary phase IIA–no hypoxia IIB–hypoxia) |
Stage III (hyperinflammation phase) |
|
|---|---|---|---|
|
Abbreviations: ARDS, acute respiratory distress syndrome; NLR, neutrophil to lymphocyte ratio; RR, respiratory rate; SIRS, systemic inflammatory response syndrome. |
|||
|
Severity of infection |
Mild (Cat A) Viral response phase |
Moderate (Cat B) Viral response phase + host inflammatory phase |
Severe (Cat C) Host inflammatory response phase |
|
Clinical symptoms |
RR < 24/min Mild constitutional symptoms–fever > 99.6°F, dry cough |
RR ≥ 24 < 30/min Without (IIA) and with (IIB) hypoxia, PaO2/FiO2 300 mm Hg |
RR ≥ 30/min Severe pneumonia, RDS, SIRS, shock, cardiac failure |
|
Investigations |
Lymphopenia; NLR > 3.13) |
Abnormal chest imaging; transaminitis, low to normal procalcitonin |
Elevated inflammatory markers (CRP, LDH, IL-6, D-dimer, ferritin, troponin, NT-pro-BNP elevation) |
|
Potential therapies |
Antivirals ± prophylactic dose of anticoagulation |
Antivirals + anti-inflammatory (steroids) ± convalescent plasma + prophylactic dose of anticoagulation |
Anti-inflammatory ± antivirals ± convalescent plasma + prophylactic/therapeutic dose of anticoagulation |
The management of COVID-19 is done in accordance with the Ministry of Health and Family Welfare, Govt. of India (updated on 3/7/2020).2
Management of Mild COVID-19 Cases (Symptomatic influenza-like illness [ILI] without Pneumonia or Lung Signs)
-
May admit those with high risk in COVID care centers (CCC), community health center, district hospitals, or home isolation.
-
Counsel the patients regarding worsening of symptoms and signs, specifically in those with comorbidities.
-
Investigations at diagnosis: CBC,–NLR, X-ray chest, CRP, AST, ALT, D-dimer, LDH.
-
Monitor pulse oximetry, temperature and respiratory rate every 2 to 4 hours or earlier if needed.
-
Symptomatic treatment with paracetamol for fever and pain; antitussives for cough.
-
Consider hydroxychloroquine (HCQ)–under strict supervision in those with comorbidities (MOHF-version 5; dated 3/7/2020).
-
If there is worsening hypoxia (if drop in SpO2) or increase in respiratory rate ≥5/min from baseline, repeat D-dimer, ABG, X-ray chest, consider giving prophylactic dose of anticoagulation, and refer to dedicated COVID health center (DCHC).3
-
An initial dose of steroid may be administered only in the presence of hypoxia (SpO2 < 94%).
Management of Moderate COVID-19 Cases (Pneumonia–Clinical or Radiological, with or without Hypoxia; respiratory rate [RR] > 24/min)
-
Admit in DCHC.
-
Monitor SpO2, at least, every 2 hours along with RR.
-
Investigations: CBC, X-ray chest, CRP, AST, ALT, D-dimer, LDH, ferritin, EKG, ABG.
-
Oxygen to be administered (target SpO2: 92–96%; 88–92% in patients with chronic obstructive pulmonary disorder [COPD]); devices to deliver oxygen maybe via nasal prongs, mask, or masks with breathing/nonrebreathing mask (NRBM); if high-flow nasal cannula (HFNC) or simple nasal cannula is used, N95 mask should be applied.
-
Promote “awake prone ventilation” (can be done in patients with oxygen requirement of > 4 L, having a normal mental status and who are able to self-prone or change position with minimal assistance).
-
Give prophylactic dose of anticoagulation if not contraindicated; if D-dimer levels are increasing, consider increasing to therapeutic doses of anticoagulation; consider imaging–computed tomography pulmonary angiography (CTPA), Doppler lower limbs and echocardiogram for thrombotic disease.
-
Administer IV methylprednisolone or dexamethasone (if oxygen requirement is increasing or inflammatory markers are elevated). Do not start steroids if patient not on oxygen. Continue to review the duration of administration, according to the clinical response.
-
Consider remdesivir (under emergency use authorization [EUA]) and convalescent plasma (off label use).
-
Repeat CRP, D-dimer, and ferritin every 48 to 72 hours.
-
Control comorbidities and consider empiric antibiotic therapy in elderly with comorbidities, immunocompromised individuals, and children < 5 years of age.
-
Close monitoring of patients with moderate COVID-19 is required for signs or symptoms like increasing oxygen requirement or hemodynamic instability; if present, shift to a DCHC.
Management of Severe COVID-19 Cases (pneumonia with RR > 30/min/acute respiratory distress syndrome [ARDS]/shock/SpO2 < 90% on right atrial [RA])
-
Admit to a DCHC.
-
Investigations: CBC, X-ray chest, CRP, AST, ALT, D-dimer, LDH, ferritin, EKG, ABG.
-
Continuous pulse oximetry and hemodynamic monitoring is mandatory.
-
Oxygen support: target SpO2 92 to 96% (88–92% in COPD); escalate oxygen therapy as required; patients may be on noninvasive ventilation (NIV), NRBM, HFNC or need to be ventilated.
-
Repeat D-dimer if escalating respiratory support needed.
-
Continue anticoagulation with prophylactic dose and increase to therapeutic dose if D-dimer levels are increasing; consider imaging–CTPA, Doppler lower limbs, and echocardiogram for thrombotic disease.
-
Give IV methylprednisolone/dexamethasone.
-
Consider giving tocilizumab (Infectious Diseases Society of America [IDSA] guidelines)4 in the context of clinical trial; rule out infection.
-
Consider remdesivir (IDSA).4
-
Prone ventilation if no contraindication (all intubated patients should also be proned).
-
Management of ARDS/shock/sepsis (choose antibiotics to cover Pseudomonas and Staph).
Recommended Dosing Schedule
HCQ: 400 mg bd X 1 day followed by 200 mg bd × 4days (avoid if QTc > 500 ms).
Remdesivir: 200 mg stat IV followed by 100 mg OD IV for 4 days (interaction with HCQ); monitor liver function test.
IV Methylprednisolone: 0.5 to 1 mg/kg for 3 to 5 days.
IV Dexamethasone: 0.1 to 0.2 mg/kg (6 mg) OD for 10 days; use prednisolone or 40 mg/day or hydrocortisone 80 mg bd if pregnant or breast feeding.
Anticoagulation preferred with enoxaparin 40 mg OD s/c or fondoparinux 2.5 mg OD s/c or unfractionated heparin 5000 units BD s/c.
Therapeutic dose of enoxaparin: 1 mg/kg BD s/c.
Tocilizumab: 400 mg stat (8 mg/kg); maximum of 2 doses.
Monitor IL-6, CRP, ferritin.
Contraindicated in people living with HIV (PLHIV), active infections, tuberculosis (TB), active hepatitis, absolute neutrophil count (ANC) < 2000/mm3 and platelet count < 1,00,000/mm3.
Convalescent plasma: 4 to 13 mL/kg (200 mL) single dose over 2 hours.
Avoid in patients with IgA deficiency or immunoglobulin allergy.
Conflicts of Interest
None declared.
References
- Siddiqui HK, Mehra M. COVID-19 illness in native and immunosupressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020 (e-pub ahead of print). doi: https://dx.doi.org/10.1016%2Fj.healun.2020.03.01
- Government of India, Ministry of Health and Family Welfare, Directorate General of Health Services (EMR Division); Version 5; dated 03.07.20. Available at: https://www.mohfw.gov.in/. Accessed July 10, 2020
- Infectious Disease Society of America Guidelines on the treatment and Management of Patients with COVID-19 (updated18/6/2020). Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed July 10, 2020







