Translate this page into:
Heart Failure in a Patient with Leprosy
Srikakulapu SriKrishna, MD Department of Cardiology, NIMS Flat 309, Sai Mithra Legacy, Kamala Puri Colony, Hyderabad, 500045 India Ssrikrishnamd@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Leprosy, also known as Hansen's disease, is a chronic infection caused by the bacteria Mycobacterium leprae and Mycobacterium lepromatosis. Though leprosy predominantly involves peripheral nerves and skin, visceral involvement was recognized as early as 1894 by Hansen and Looft. It is known that the bacilli can lie dormant in some tissues of the body long after the skin smears have become negative. We describe a case of leprosy in an Indian male who presented to us with heart failure. This is likely the first report of showing the possible association of leprosy with heart failure from India. The cardiovascular involvement in leprosy is briefly reviewed.
Keywords
heart failure
leprosy
Introduction
Leprosy is a chronic granulomatous infectious disease, the causative organisms being Mycobacterium leprae and Mycobacterium lepromatosis. It has a long incubation period and a contagious disease. The mycobacterium preferably infects peripheral tissue, as it survives at 30°C rather than 37°C. Therefore, it affects the skin, peripheral nerves, and the mucosa of the upper airways. It also effects bones, some viscera, and other tissues.
We describe a case of leprosy in an Indian male who presented to us with heart failure. This is likely the first report of showing the possible association of leprosy with heart failure from India.
Case Report
A 50-year-old man, ex-smoker and ex-alcoholic without a prior cardiovascular history, presented to the emergency room complaining about worsening breathlessness over the last 7 days with two episodes of paroxysmal nocturnal dyspnea during this time. He did not complain of chest pain, palpitations, or syncope.
On physical examination, the patient was tachypnea for minimal activities (respiratory rate 30/min–Sat02 87% in room air); heart rate was 120 bpm and blood pressure was 140/80 mm Hg. Jugular venous pulse was elevated, fine crepitation’s were present in lower two-thirds of lung fields; on auscultation, he had s3, and a pan systolic murmur of grade 3 at the apex; no peripheral edema was appreciable.
The patient had dry fissures on the skin of both hands, partial necrosis of skin on fingertips and partial resorption of lips. His peripheral nerves (ulnar, peroneal) were thickened. His skin lesions showed definite sensory loss. His physical features are consistent with borderline tuberculoid leprosy.
Chest X-ray was suggestive of congestion and cardiomegaly. Blood cell counts were as follows: total leukocyte count, 21,800/µL (neutrophils: 94%, lymphocytes: 4%, and monocytes: 2%), hemoglobin, 10.1 g/dL; renal function tests were as follows blood urea, 111 mg/dL and serum creatinine, 2.7 mg/dL. Liver function tests were as follows: serum glutamic-oxaloacetic transaminase, 898 U/L; serum glutamic pyruvic transaminase, 581U/L; total bilirubin, 0.6 mg/dL; total proteins, 7.8; albumin, 3.3 g/dL; prothrombin time, 15 s; international normalized ratio, 1.4. Erythrocyte sedimentation rate was 59; troponin I was normal; and brain natriuretic peptide was 1750 pg/mL.
Electrocardiogram was suggestive of sinus tachycardia, left atrial enlargement, left ventricular hypertrophy with volume overload features with relatively low voltage in the limb leads if compared with precordial ones and T inversions in 1, AVL, v4, v5, and v6 (Fig. 1).
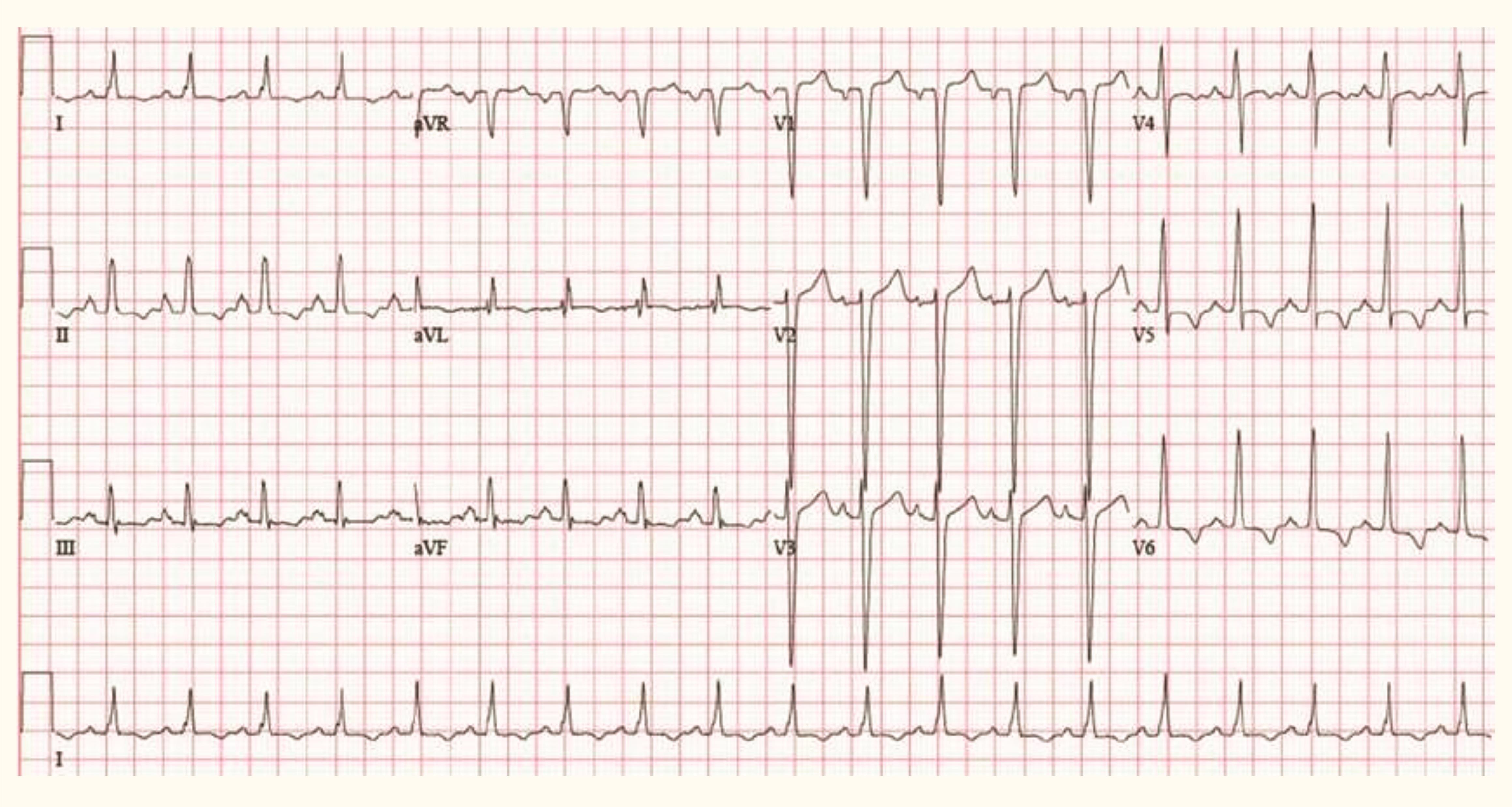
-
Fig. 1 Electrocardiogram of the patient showing features of dilated cardiomyopathy.
Fig. 1 Electrocardiogram of the patient showing features of dilated cardiomyopathy.
Echocardiography revealed a dilated left ventricle (6.4 cm * 5.4 cm), dilated left atrium (4.6 cm) with severe left ventricular dysfunction (left ventricular ejection fraction 20%) and global hypokinesia with grade 3 diastolic dysfunction and moderate functional mitral regurgitation; moreover, he had good right ventricular function with tricuspid annular plane systolic excursion of 1.9 with mild pulmonary hypertension (Fig. 2).
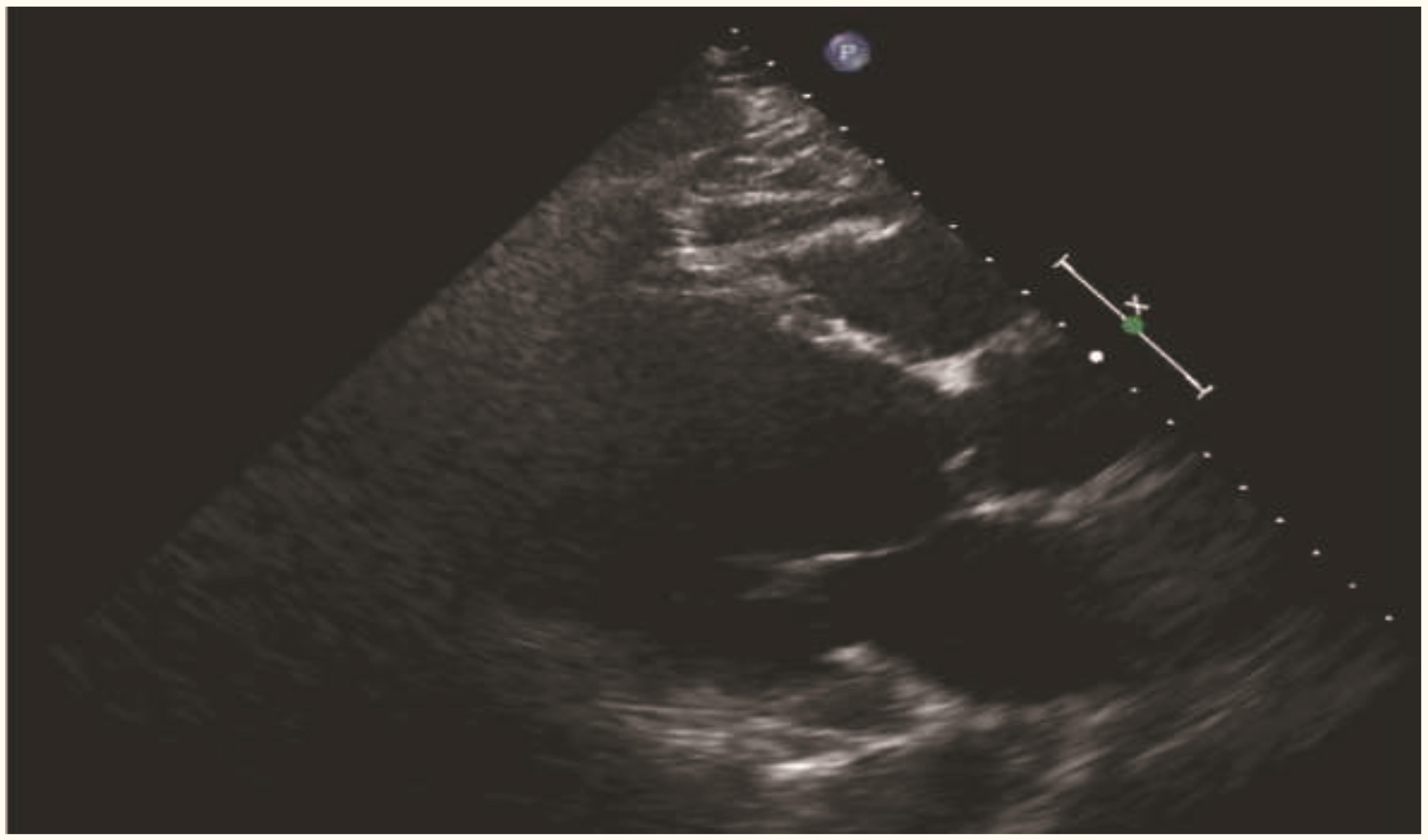
-
Fig. 2 Two-dimensional echocardiography of the patient showing dilated left ventricle and left atrium.
Fig. 2 Two-dimensional echocardiography of the patient showing dilated left ventricle and left atrium.
Coronary angiography was done and was suggestive of normal epicardial coronaries.
The patient was stabilized with inotropes, furosemide, and nitroglycerin infusion. The patient improved clinically, his breathlessness decreased and urine output increased. A low dose of carvedilol was started before being discharged for follow-up. The patient was planned for a positron emission tomography scan and endomyocardial biopsy later.
Discussion
This article reports on the diagnosis of dilated cardiomyopathy in a patient with leprosy. Although the patient had other risk factors for developing dilated cardiomyopathy, such as alcoholism, smoking, and hypertension, the combined diagnosis of leprosy and heart failure might be significant in this patient. The patient stopped consuming alcohol and abstained from smoking from past 5 years; they might not be important in the etiology of this patient’s heart failure.
Mathur et al described two cases of lepromatous leprosy that presented with heart disease: one with ischemic heart disease and the other with cardiomyopathy in 1976.2 No other case reports showing the association of leprosy with heart failure has been published since. It is difficult to describe the heart failure in this patient with leprosy but a possible association can be made.
The heart could be involved (i) due to the disease itself, (ii) due to drugs used, (iii) due to metabolic alterations in the body due to disease, and (iv) due to denervation or disautonomia.
In the various autopsy studies done in leprosy patients by Sakurai et al3 and Desikan and Job,4 no granulomata specific to leprosy was demonstrated in the heart or major blood vessels. However, Mitsuda and Ogawa5 while reporting on autopsies of leprosy patients observed that the heart had no lepromatous infilterates but had structures that could be atheroma or some form of endocardial elastoses.
Miguel and Miro6 while studying the circulatory function of 38 leprosy patients found that five had myocarditis. Various investigations performed in this documented report including histological examination of cases that were autopsied indicate that leprosy does not selectively involve the heart. Myocarditis is thought to be due to nonspecific infiltration and to hyaline degeneration sometimes encountered in autopsies. In these cases, a condition of generalized amyloidosis was found and the hyaline degeneration related to amyloidosis may be regarded in the heart as an expression of generalized metabolic disturbance caused by leprosy.
The accumulated literature on three commonly used antileprosy drugs clofazimine, rifampicin, and dapsone-induced adverse effects does not contain any reference to cardiotoxicity so it is unlikely that these drugs are responsible for this patient’s heart failure
Leprosy patients are at risk of cardiac autonomic neuropathy. Solanki et al observed a high prevalence of abnormally prolonged QTc with female preponderance in middle-aged leprosy patients having predominantly multibacillary bacteriological profile. Leprosy imposed six times risk for prolonged QTc as great as that for the age- and gender-matched healthy controls. QTc value, a feature of cardiac autonomic neuropathy, did not correlate with age, duration, or clinical profile for leprosy and peripheral neuropathy.7
Treu et al showed lepromatous leprosy causes severe microvascular dysfunction and significant alterations in capillary structure.8 Since microvascular dysfunction is a systemic process that occurs similarly in the entire body,9 it is possible to consider that the impairment of microvascular function observed in the skin microvasculature also occurs in the coronary microcirculation, which results in heart failure.
Though the coronaries are normal in this patient, a chronic microvascular dysfunction that has caused myocardial failure can be considered.
Conclusion
There have been only a few case reports of patients with leprosy presenting with heart failure, our case being one of them. Leprosy can have varied and rare presentations. This case highlights the cardiac failure as a part of multisystem involvement in Hansen’s disease. Treatment of dilated cardiomyopathy caused by leprosy is the same as standard heart failure therapy with no role of immunosuppressants and steroids.
Conflicts of Interest
None declared.
References
- Die lepra vom klinischen and pathologidch anatomischen standpunkcte. Fiher and Co; Bristol John Wright and Co; 1894. 1895. p. :p 45. ed. Translated by
- [Google Scholar]
- Congestive heart failure in two patients of lepromatous leprosy. Lepr India. 1976;48(01):75-80.
- [Google Scholar]
- Histopathological findings in 115 corpses of leprosy. (O) Nagashima Arch LeprosyInt J Lepr. 19641964;6(O) Nagashima Arch LeprosyInt J Lepr. 19641964;32:17-22. (A). 349
- [Google Scholar]
- A review of postmortem findings in 37 cases of leprosy. Int J Lepr Other Mycobact Dis. 1968;36(01):32-44.
- [Google Scholar]
- Condition of heart in leprosy. (O) YontillesInt J Lepr. 19491951;2(O) YontillesInt J Lepr. 19491951;19:202-226. (A). 258
- [Google Scholar]
- Evaluation of cardiac autonomic status using QTc interval in patients with leprosy. Asia Pac J Clin Trials Nerv Syst Dis. 2016;1:144-148.
- [Google Scholar]
- Structural and functional changes in the microcirculation of lepromatous leprosy patients - Observation using orthogonal polarization spectral imaging and laser Doppler flowmetry iontophoresis. PLoS One. 2017;12(04):e0175743.
- [Google Scholar]
- Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J. 2018;39(46):4086-4097.
- [Google Scholar]







