Translate this page into:
Endocrine Disruptors and the Heart: Unraveling the Cardiovascular Impact
*Corresponding author: Vanishri Ganakumar, Department of Endocrinology, Jawaharlal Nehru Medical College, KLE Dr Prabhakar Kore Hospital, Belgaum, Karnataka, India. drvanishriganakumar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ganakumar V, Sruthi K, Ghatnatti VB, Goroshi M. Endocrine Disruptors and the Heart: Unraveling the Cardiovascular Impact. Indian J Med Sci. 2024;9:230-40. doi: 10.25259/IJCDW_68_2024
Abstract
Endocrine disrupting chemicals (EDCs) are environmental contaminants that interfere with the hormonal system, posing significant risks to human health. Found in everyday items such as plastics, pesticides, cosmetics, and industrial materials, EDCs include both persistent chemicals, for example, dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), and per- and polyfluoroalkyl substances (PFAS) and non-persistent ones, for example, bisphenol A (BPA), phthalates, and parabens. Mechanistically, EDCs mimic or block natural hormones, affecting processes such as glucose metabolism, lipid metabolism, and insulin signaling. These disruptions elevate the risk of obesity, type 2 diabetes mellitus, metabolic syndrome, and cardiovascular diseases, especially when exposure occurs during prenatal and early life stages. The detrimental cardiovascular impact of EDCs extends beyond their diabetogenic and obesogenic effects. EDCs such as BPA and heavy metals disrupt estrogen and androgen signaling, leading to hypertension, endothelial dysfunction, and arterial stiffness. In addition, EDCs further promote oxidative stress, which contributes to atherosclerosis and cardiovascular events. EDCs significantly impact reproductive health, causing precocious puberty, infertility, polycystic ovarian syndrome, endometriosis, and uterine fibroids in females and precocious puberty, delayed puberty, and infertility in males. The health ramifications of EDCs extend beyond the individual and can affect ensuing generations. EDCs cause epigenetic changes that can be passed down to future generations, compounding long-term impact on quality of life and healthcare spending. Intrauterine EDC exposure is associated with profound impact on fetal growth, potentially leading to low birth weight and intrauterine growth restriction, which predispose these individuals to life-long metabolic and cardiovascular challenges. Mitigation of EDC exposure requires a comprehensive prevention strategy to minimize the widespread health impacts, starting from the individual and the family unit. These include making simple swaps in daily life such as minimizing use of plastic, processed food items, scrutinizing cosmetics, and paints for possible presence of EDCs and avoiding exposure to direct and second-hand smoking. However, broader regulatory actions need to be initiated on a global level to implement stricter safety standards, minimize production of EDCs and their entry into the ecosystem and exploring safer alternatives.
Keywords
Endocrine disrupting chemicals
Metabolic syndrome
Cardiovascular disease
Obesity
Insulin resistance
INTRODUCTION
“Endocrine disruptors” are exogenous compounds that negatively impact endocrine functions and demonstrate a definite cause-and-effect relationship in individuals, offspring, or subpopulations exposed to them.[1,2] Endocrine society defines EDC as “An Exogenous chemical, or mixture of chemicals, that interfere with hormone action.” They can originate from natural sources such as plants (phytoestrogens), animals, or humans. More commonly, endocrine disruptors are of environmental origin , and are otherwise known as endocrine-disrupting chemicals (EDCs). Exposure to EDCs can occur through inhalation (e.g., plasticizers), oral (e.g., food), dermal (e.g., cosmetics), embryonic transfer (e.g., from mother), and transfer to offspring through breast milk.
The publication of Rachel Carson’s Silent Spring in 1962 was a pivotal turning point that brought attention to the environmental and health impacts of synthetic pesticides, particularly dichlorodiphenyltrichloroethane (DDT). It highlighted the harmful effects on wildlife, particularly bird populations, and raised concerns about human cancer risks. This led to the nationwide ban on DDT in the United States and the creation of the Environmental Protection Agency.[3] The year 1971 was pivotal for the discovery of transplacental carcinogenic effect of diethylstilbestrol (DES), a synthetic estrogen given to pregnant women to prevent miscarriages at the time, leading to cancer and reproductive issues in the daughters of women who had taken the drug during pregnancy. This catastrophe emphasized the dangers of endocrine disruptors, especially during pregnancy.[4] In 1991, the term “Endocrine Disruptor” was coined during the Wingspread Conference, recognizing chemicals that interfere with the hormonal system.[5]
EDCs have been increasingly recognized in our ecosystem since the discovery of their harmful effects. Their widespread presence in the environment raises major concerns about the impact of EDCs on human health, especially their role in the development of chronic conditions such as diabetes, obesity, and cardiovascular disease (CVD).[6-9] The health implications of EDCs also span across generations. There is mounting evidence linking preterm delivery and intrauterine growth restriction to in utero exposure to EDCs.[10-13]
Given the high prevalence of type 2 diabetes mellitus (T2DM) and macrovascular disease in contemporary society, it is imperative to understand the associations between environmental contaminants and these disease states to develop successful preventative measures and explore novel therapeutic strategies.
COMMON EDCs AND WHERE THEY ARE FOUND
EDCs are pervasive in nature and include phenols, phthalates, parabens, flame retardants, heavy metals, pesticides, perfluorinated chemicals, ultraviolet filter components, triclosan, and organochlorines. Among these, of particular concern are polychlorinated biphenyls (PCBs), polybrominated biphenyls, dioxins, bisphenols, DDT, vinclozolin, DES, and heavy metals, such as cadmium, mercury, arsenic, lead, manganese, and zinc. These chemicals are found in industrial products, agricultural pesticides, plastics, packaged foods, cosmetics, and pharmaceuticals.[14-16] EDCs are thought to number more than 4000 currently and their presence in the environment is being increasingly recognized. Pertinent EDCs present in the environment and their health implications are been listed in Table 1.[17]
| Common EDCs | Sources | Health implications |
|---|---|---|
| DDT, chlorpyrifos, atrazine, 2,4 D, glyphosate | Pesticides | Obesity Metabolic syndrome Type 2 diabetes Reproductive toxicity Neurotoxicity |
| Lead, phthalates, cadmium | Children’s toys, feeding bottles, accessories, batteries | Neurotoxicity Impaired development Oxidative stress Metabolic disruption Cardiovascular disease |
| PCBs, dioxins | Industrial solvents | Immune dysfunction Thyroid dysfunction Developmental delay Malignancy |
| BPA, Phthalates, Phenol | Plastics, food storage materials, reusable bottles | Insulin resistance Obesity Cardiovascular disease Hormonal imbalance |
| Brominated flame retardants, PCBs | Electronics, furniture and Building materials | Developmental delay Thyroid dysfunction Reproductive toxicity |
| Phthalates, parabens | Personal care products, medical tubing, plastic food containers, plastic wraps | Obesity Reproductive disorders Metabolic syndrome |
| Triclosan | Anti-bacterial soaps, antiseptics | Thyroid dysfunction Antimicrobial resistance |
| Perfluorochemicals | Textiles, clothing, non-stick cookware | Hepatotoxicity Immune dysfunction Reproductive toxicity Thyroid dysfunction |
BPA: Bisphenol A, DDT: Dichlorodiphenyltrichloroethane, EDC: Endocrine-disrupting chemicals, PCBs: Polychlorinated biphenyls
CLASSIFICATION OF EDCs
EDCs can be classified as persistent EDCs and non-persistent EDCs. DDT, dichlorodiphenyl dichloroethylene (DDE), PCBs, polybrominated diphenyl ether, and per- and polyfluoroalkyl substances (PFAS) are persistent EDCs, they can persist in the environment for decades.[18] This can occur secondary to bioamplification or biomagnification, which refers to an increase in the concentration of a substance as it moves up the food chain. This occurs when a pollutant is persistent; it cannot be, or is very slowly, broken down by natural processes. Hence, these persistent pollutants are transferred up the food chain faster than they are broken down. The extended latency interval between illness and exposure makes it challenging to establish a definitive cause-and-effect relationship.[19]
Phenols, phthalates, parabens, acrylamide, and solvents are non-persistent EDCs. However, they can bioaccumulate in adipose tissue and lead to adverse health outcomes.[20] Bioaccumulation refers to the process where a concentration of a substance, typically lipophilic rather than hydrophilic, builds up in the tissues because it is absorbed more quickly than eliminated (eg: DDT in adipose tissues). EDCs can also be classified as per their health impact such as those causing cardiometabolic effects, effects on reproductive axis, etc., which are discussed in detail in the subsequent sections.
MECHANISM OF ACTION OF EDCs
EDCs can interfere with hormonal signaling in the body through various mechanisms, primarily by functioning as receptor agonists or antagonists. As receptor agonists, EDCs mimic the structure of natural hormones, binding to hormone receptors and enhancing or initiating hormonal responses. Conversely, as receptor antagonists, EDCs bind to the same receptors but block their activity, preventing the natural hormone from exerting its effects.[20] For nuclear hormone receptors, this interference extends to disrupting the receptor-hormone complex’s ability to bind to DNA, thereby altering the transcription of gene cascades and affecting downstream biological processes. These actions underline the significant impact of EDCs on endocrine system regulation and gene expression. The ways in which EDCs interact with cell surface and nuclear receptors to cause downstream effects are demonstrated in Figure 1.
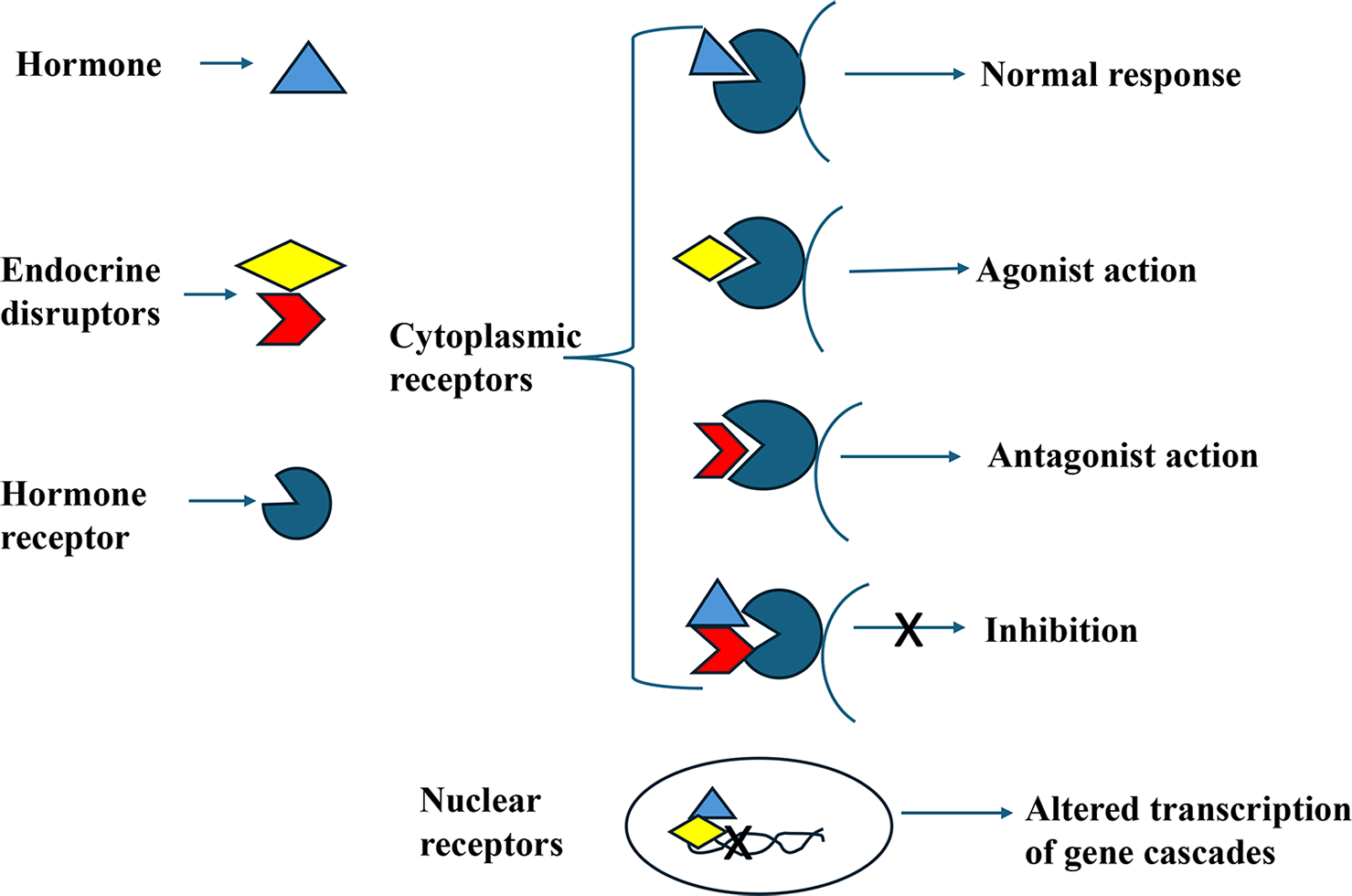
- Mechanisms of action of endocrine disrupting chemicals.
PATHOPHYSIOLOGICAL MECHANISMS OF EDCs AFFECTING CARDIOVASCULAR SYSTEM
EDCs can impact cardiovascular function through several molecular and cellular mechanisms which primarily involve the disruption of hormonal signaling pathways. The major mechanisms by which EDCs influence cardiovascular health have been summarized in Figure 2, and discussed in detail as follows:
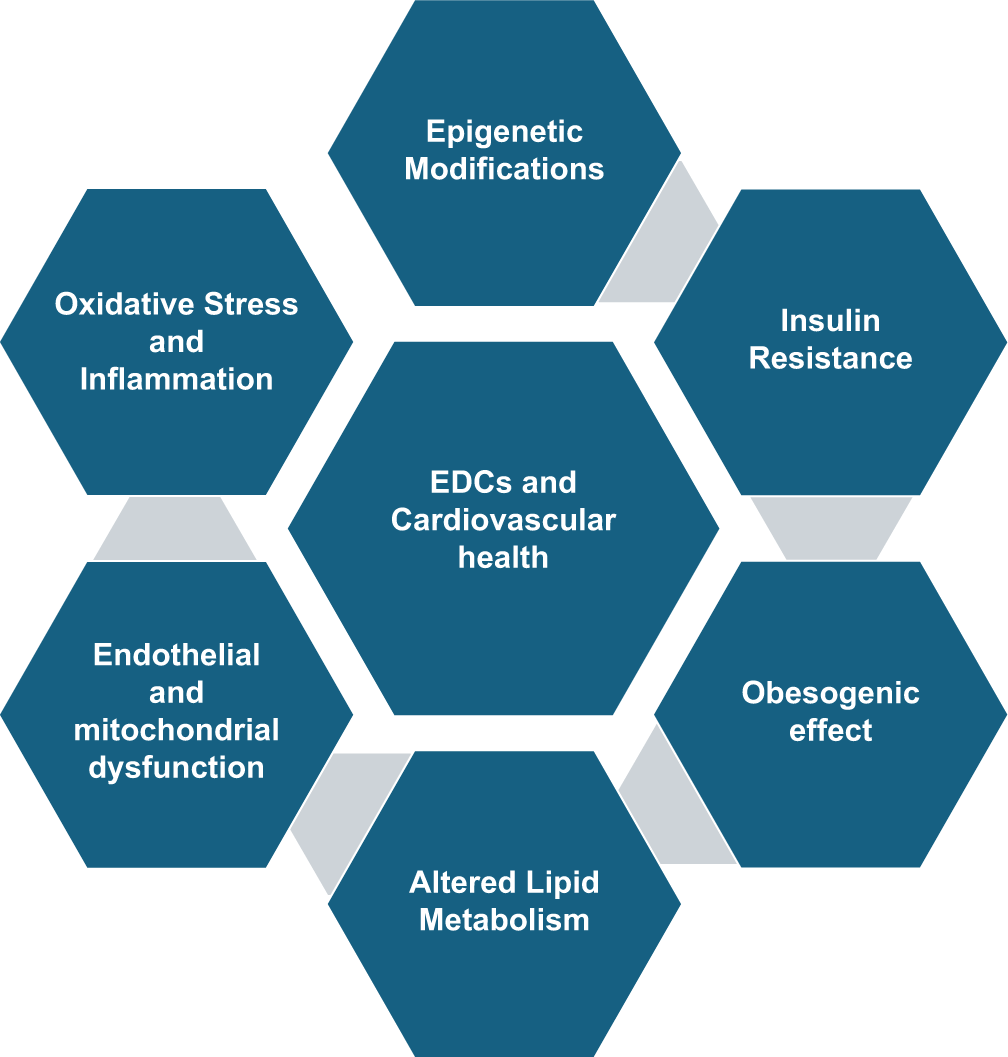
- Mechanisms of impact of endocrine disrupting chemicals on cardiovascular health.
Hormone Receptor Binding and Activation: Many EDCs either mimic or block the actions of natural hormones by interacting with hormone receptors, which play a key role in cardiovascular functions. Estrogens support cardiovascular health by regulating vascular tone, cholesterol metabolism, and endothelial function. Bisphenol A (BPA) can bind to estrogen receptors, leading to abnormal signaling, which can result in arterial stiffness, endothelial dysfunction, and altered lipid metabolism.[21] EDCs, such as certain pesticides, can disrupt androgen signaling, which may impair vascular function and elevate the risk of hypertension (HTN) and CVD in males. PCBs can interfere with thyroid hormone signaling, leading to changes in heart rate, cholesterol metabolism, and an increased risk of atherosclerosis and arrhythmias.[15]
Oxidative Stress and Inflammation: Many EDCs elevate oxidative stress levels in cardiovascular tissues. Excessive production of reactive oxygen species damages proteins, lipids, and endothelial cells, contributing to HTN and atherosclerosis.[9] Chronic vascular inflammation may also arise from EDC-induced activation of pro-inflammatory signaling pathways, such as the nuclear factor kappa-light-chain-enhancer of activated B-cells pathway. This persistent inflammation plays a crucial role in the progression of atherosclerosis, cardiac remodeling, and endothelial dysfunction.[22]
Endothelial Dysfunction: EDCs like BPA reduce nitric oxide (NO) bioavailability by inducing oxidative stress or disrupting the NO synthase (NOS) pathway, which impairs vasodilation and elevates blood pressure.[23] Prolonged exposure to EDCs can compromise the integrity of endothelial cells, increasing vascular permeability and facilitating the development of atherosclerosis.[24]
Altered Lipid Metabolism: EDCs, including phthalates and BPA, can interfere with peroxisome proliferator-activated receptors (PPARs) which leads to triglyceride accumulation, decreased high-density lipoprotein (HDL), and increased low-density lipoprotein (LDL) cholesterol, all of which contribute to the development of atherosclerosis.[25]
Insulin Resistance (IR): EDCs such as phthalates and BPA can disrupt insulin signaling, leading to IR.[7,26] Furthermore, EDCs may impair cellular glucose uptake, increasing the risk of hyperglycemia and diabetes.[27]
Obesogens: Some EDCs, known as “obesogens,” promote adipogenesis, resulting in obesity, which is a major risk factor for CVD, HTN and heart failure.[28] Exposure to obesogens during sensitive periods of early development predisposes individuals to weight gain due to changes in metabolic set-points.[29,30]
Mitochondrial Dysfunction: EDC-induced mitochondrial dysfunction can lead to the apoptosis of cardiomyocytes and endothelial cells, which contributes to cardiac remodeling and loss of vascular integrity.[31]
Epigenetic Modifications: EDCs can alter DNA methylation patterns and histones, affecting the expression of genes involved in regulating blood pressure, cholesterol metabolism, and vascular function.[32] These epigenetic modifications may be passed down to future generations, potentially increasing the risk of CVD in children who have not been directly exposed to the chemicals.[33]
Miscellaneous actions: EDCs, such as BPA and certain heavy metals, can elevate angiotensin II levels, contributing to HTN and cardiac hypertrophy.[34] In addition, some EDCs can elevate aldosterone secretion, leading to HTN and a higher risk of heart failure by promoting sodium and water retention.[35] EDCs, such as lead and cadmium, can disrupt calcium homeostasis and cause arrhythmias, poor cardiac muscle contractility, and impaired vascular function by interfering with calcium signaling.[36]
SPECIFIC EDCs AND CARDIOVASCULAR EFFECTS
Lead
Lead accumulates in soft tissues and bones, where it can persist for over 25 years, posing long-term health risks. It promotes free radical damage, disrupts NO signaling, and induces inflammation, all of which contribute to a higher risk of CVD and increased cardiovascular mortality, particularly in individuals with occupational lead exposure.[37,38]
Cadmium
Cadmium exposure induces oxidative stress, endothelial dysfunction, and inflammation. Large epidemiological studies have consistently linked cadmium exposure to an increased risk of HTN, myocardial infarction (MI), CVD, and higher mortality rates.[39] Cadmium has also been associated with higher prevalence of atherosclerotic plaque formation.[40,41] In addition, cadmium presence in carotid plaques is associated with increased plaque vulnerability, further elevating the risk of cardiovascular events.[42]
BPA
BPA, a monomer used in polycarbonate plastics, is widely prevalent in the environment.[43] A study utilizing data from the US 2003–2016 National Health and Nutrition Examination Surveys (N = 11,857) found that individuals in the highest quartile of urinary BPA levels had a higher incidence of MI and stroke. In addition, increased urinary BPA was associated with a 13% higher risk of developing CVDs.[44] Higher urinary BPA levels have been associated with increased risk of HTN, obesity, and type 2 diabetes (T2D).[45,46]
Phthalates
Phthalates are widely used in various consumer goods, plastics, and medical equipment.[47] Phthalate metabolites have been associated with IR and abdominal obesity, increased cardiovascular mortality in affected populations.[48,49] Proposed mechanisms for phthalate-associated metabolic disruption include alterations in hepatic metabolism, enhanced adipose tissue differentiation and dysbiosis of gut microbiota.[50-52] Phthalates may also elevate blood pressure through altered phosphorylation of endothelial NOS and induction of the angiotensin type 1 receptor.
Pesticides
According to a recent meta-analysis by the National Toxicology Program (NTP), there is sufficient evidence to support a positive association between T2DM and persistent organic pollutants (POPs). These diabetogenic POPs include the pesticide DDT and its metabolite DDE, along with pollutants from the dioxin and PCB families.[53] Organochlorine pesticides and their metabolites (e.g., DDE) as well as various PCB congeners have been positively associated with obesity, abdominal adiposity, and components of the metabolic syndrome (MS).[54-56] The Hispanic Community Health Study, which included 7404 adult Hispanic and Latino participants, investigated the effects of occupational exposure to pesticides and solvents on cardiovascular health, and found that individuals exposed to these substances had a higher risk of HTN and dyslipidemia. In addition, these exposures were linked to a two-fold increase in the risk of CVD and a sixfold higher risk of cerebrovascular disease.
Arsenic
NTP identified a potential link between arsenic, a common groundwater contaminant, and the development of diabetes with stronger associations observed at higher exposure levels.[57] Inorganic arsenic has been positively correlated with carotid intimal medial thickness and elevated serum levels of matrix metalloproteinase-9, a biomarker for CVD.[58,59] In a study on endemic arsenic exposure in Bangladesh, arsenic levels were found to be associated with lower levels of HDL and an increase in atherogenic oxidized LDL, despite lower total levels of LDL and total cholesterol (TC).[60]
Particulate matter (PM)
PM, a component of tobacco smoke and ambient air pollution, is linked to significant long-term cardiovascular and metabolic consequences.[61-64] Exposure to PM of either the 2.5 μm (PM2.5) or 10 μm (PM10) size has been associated with impaired insulin sensitivity or an increased incidence of diabetes.[65] Tobacco smoke remains a significant risk factor for atherosclerosis, CVD, and metabolic dysfunction.[66]
Hence, there is ample evidence from population-based data to support the idea that numerous environmental pollutants have the potential capacity to promote the development of diabetes and other risk factors associated with atherosclerosis, ultimately predisposing the individual to develop CVD. Pertinent EDCs with adverse cardiovascular effects are shown in Table 2.
| EDC | Source | Cardiometabolic effects | Mechanisms |
|---|---|---|---|
| Lead | Occupational exposure, environmental sources | • Cardiovascular disease (CVD) • Increased cardiovascular mortality |
• Promotes free radical damage • Disrupts nitric oxide (NO) signaling • Inflammation |
| Cadmium | Smoking, industrial waste | • Hypertension (HTN) • Myocardial infarction (MI) • CVD • Higher mortality rates |
• Oxidative stress • Endothelial dysfunction • Inflammation • Atherosclerotic plaque formation and Vulnerable carotid plaques |
| Bisphenol A (BPA) | Plastics, food packaging, environment | • MI • Stroke • Hypertension • Obesity • Type 2 diabetes • CVD |
• Disrupts estrogen signaling • Promotes insulin resistance • Alters lipid metabolism • Oxidative stress |
| Phthalates | Plastics, consumer goods, medical equipment | • HTN • Cardiovascular mortality • Metabolic syndrome |
• Decreases NO bioavailability • Induces angiotensin type 1(AT1) receptor • Disrupts lipid metabolism • Gut microbiota dysbiosis |
| Pesticides e.g., DDT, DDE | Agriculture, Environmental residues | • HTN • Dyslipidemia • Type 2 diabetes • Obesity • 2-fold increase in CVD risk • 6-fold higher risk of cerebrovascular disease |
• Through persistent organic pollutants (POPs), • Impacts lipid profiles and metabolic regulation |
| Arsenic | Ground water contamination | • Elevated CVD risk | • Increased carotid intimal medial thickness (cIMT), atherosclerosis • Lower HDL, higher oxidized LDL • Increased serum matrix metalloproteinase-9 • Promotes oxidative stress |
| Particulate Matter (PM) | Air pollution, tobacco smoke, environment | • Long-term CVD risks • HTN • Type 2 Diabetes • Atherosclerosis |
• Increases inflammation • Oxidative stress • Impaired insulin sensitivity • Impacts lipid and glucose metabolism |
DDT: Dichlorodiphenyltrichloroethane, DDE: Dichlorodiphenyl dichloroethylene, EDC: Endocrine-disrupting chemicals, HDL: High-density lipoprotein, LDL: Low-density lipoprotein
DIABETOGENIC AND OBESOGENIC EFFECTS OF EDCS
The Parma Consensus 2015 introduced the “Metabolic Disruptor Hypothesis,” which explains how EDCs interfere with human metabolism, contributing to disorders such as obesity, type 2 diabetes, and MS. EDCs disrupt the hypothalamus, altering gene expression and leptin signaling, leading to metabolic dysregulation. In adipose tissue, they promote fat accumulation and IR. In the liver, EDCs cause epigenetic changes, promoting steatosis and lipogenesis. EDCs also impair insulin production in the pancreas, causing oxidative stress and β-cell exhaustion. In skeletal muscle, EDCs disrupt insulin signaling, impair glucose uptake, and reduce antioxidant defenses. In addition, EDCs affect the gut microbiota, altering its composition and contributing to metabolic disorders. Together, these disruptions lead to conditions such as obesity, MS, non-alcoholic fatty liver disease, and T2D, illustrating the broad impact of EDCs on human health.[67]
Evidence summarizing the metabolic implications of EDCs have been discussed below:
EDCs and risk of obesity
Studies such as the Nurses’ Health Study, Women’s Health Initiative, and Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study found an association between urinary concentrations of phthalate metabolites and weight gain.[68] The POUNDS-LOST Trial showed that PFAS exposure was associated with reduced resting metabolic rate.[69] A study involving 2719 individuals found that higher concentrations of Di-(2-ethylhexyl) phthalate (DEHP) were associated with obesity with an odds ratio of 2.2 for MS.[70]
EDCs and risk of T2D
Higher urinary BPA levels are associated with IR and hyperinsulinemia with strongest link with diabetes risk.[7,70] A meta-analysis of seven studies confirmed an association between phthalate exposure and increased T2D risk.[71] Reduced beta-cell function is linked to childhood exposure of PFAS.[69]
EDCs and risk of gestational diabetes mellitus (GDM)
The National Institute of Child Health and Human Development (NICHD) Fetal Growth Study and a meta-analysis of 25 studies found that several congeners of PCBs, PFAS, and PBDEs were associated with increased risk of GDM.[72,73] Phthalate metabolites were correlated with higher glucose levels and an increased risk of GDM.[74]
TRANSGENERATIONAL IMPLICATIONS OF EDCs
The Developmental Origins of Health and Disease hypothesis posits that exposure to environmental factors, including EDCs, during critical periods of prenatal and early life development can result in permanent changes in the body’s structure, physiology, and metabolism, which can predispose individuals to chronic conditions such as CVDs, diabetes, and obesity later in life. Important transgenerational implications of EDCs have been summarized below:
Risk of low birth weight (LBW)
A California study involving 295,387 pregnant women found an association between pesticide exposure and LBW.[75] A meta-analysis of 24 studies revealed that birth weight decreased by 10.5 g for each ng/mL increase in perfluorooctanoic acid (PFOA) concentration in maternal or umbilical cord blood.[76]
Preterm birth
The LIFECODES study highlights the significant impact of phthalates and PFOA, on birth outcomes, with a strong link between phthalate exposure and preterm birth.[77]
Childhood adiposity
Childhood adiposity has been strongly linked to prenatal exposure to various EDCs. A meta-analysis of 10 cohort studies found that each 1 ng/mL increase in maternal blood levels of PFOA correlated with a 0.10 unit rise in body mass index Z-score and a 25% increase in the prevalence of overweight children.[78] The Project Viva study found that prenatal and mid-childhood exposure to PFAS was linked to higher TC, triglycerides, and liver function tests in boys at age 8.[79] Prenatal exposure to BPA has been associated with an increased risk of childhood obesity across several birth cohorts, contributing to hyperleptinemia, elevated blood pressure, and early weight gain.[80-83] The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study in Italy found that prenatal exposure to phthalates, phenols, or DDE was linked to higher obesity rates at ages 5 and 12.[84]
BPA mimics estrogen, activates PPARγ, and promotes differentiation of preadipocytes into mature adipocytes, prenatal exposure alters metabolic “set points,” affects gene expression through epigenetic modifications, and disrupts hypothalamic leptin signaling, leading to overeating. It also induces IR, glucose intolerance, and fat accumulation while increasing oxidative stress and inflammation, creating an obesogenic environment.
Hence, the metabolic effects of EDCs are not only limited to the index exposure but also span across generations, underlining the importance of exposure prevention especially at critical time points such as during growth, puberty, and pregnancy.
EFFECTS OF EDCS ON REPRODUCTIVE HEALTH
EDCs significantly impact female reproductive health, causing infertility, hormonal imbalances, polycystic ovarian syndrome, endometriosis, and uterine fibroids. These effects, coupled with rising EDC exposure, contribute to declining global fertility rate.[85] EDCs adversely affect male reproductive health, causing precocious puberty, delayed puberty, and infertility. These effects are linked to exposure to phthalates, PCBs, pesticides, pharmaceuticals, and other toxic substances.[86]
HOW TO AVOID EXPOSURE TO EDCs?
EDCs have well-documented negative health consequences, especially on the cardiovascular, metabolic, and reproductive systems; thus, there is an urgent need to minimize exposure. Protecting vulnerable groups, such as children and pregnant women, is all the more critical to minimize their effects on current and future generations. The pervasive nature of EDCs makes this task very challenging, and hence, proactive measures must be implemented both at individual level, through lifestyle changes to reduce exposure and at the regulatory level, with strict policies and enforcement by concerned authorities to mitigate these risks.
Strategies to mitigate EDC exposure at individual level:
Avoiding smoking and secondhand smoke:[14]
-
Food handling:
Checking ingredient list in cosmetics and skin care products. Choosing products that are labeled as “paraben-free” or “phthalate-free”[92]
Avoiding pesticide use around home[93]
Using volatile organic compound-free and water-based paints[94]
Implementing the tenets of 4 R’s: Reduce, reuse, recycle and recover.
Simple swaps to minimize EDC exposure in daily life are shown in Figure 3.
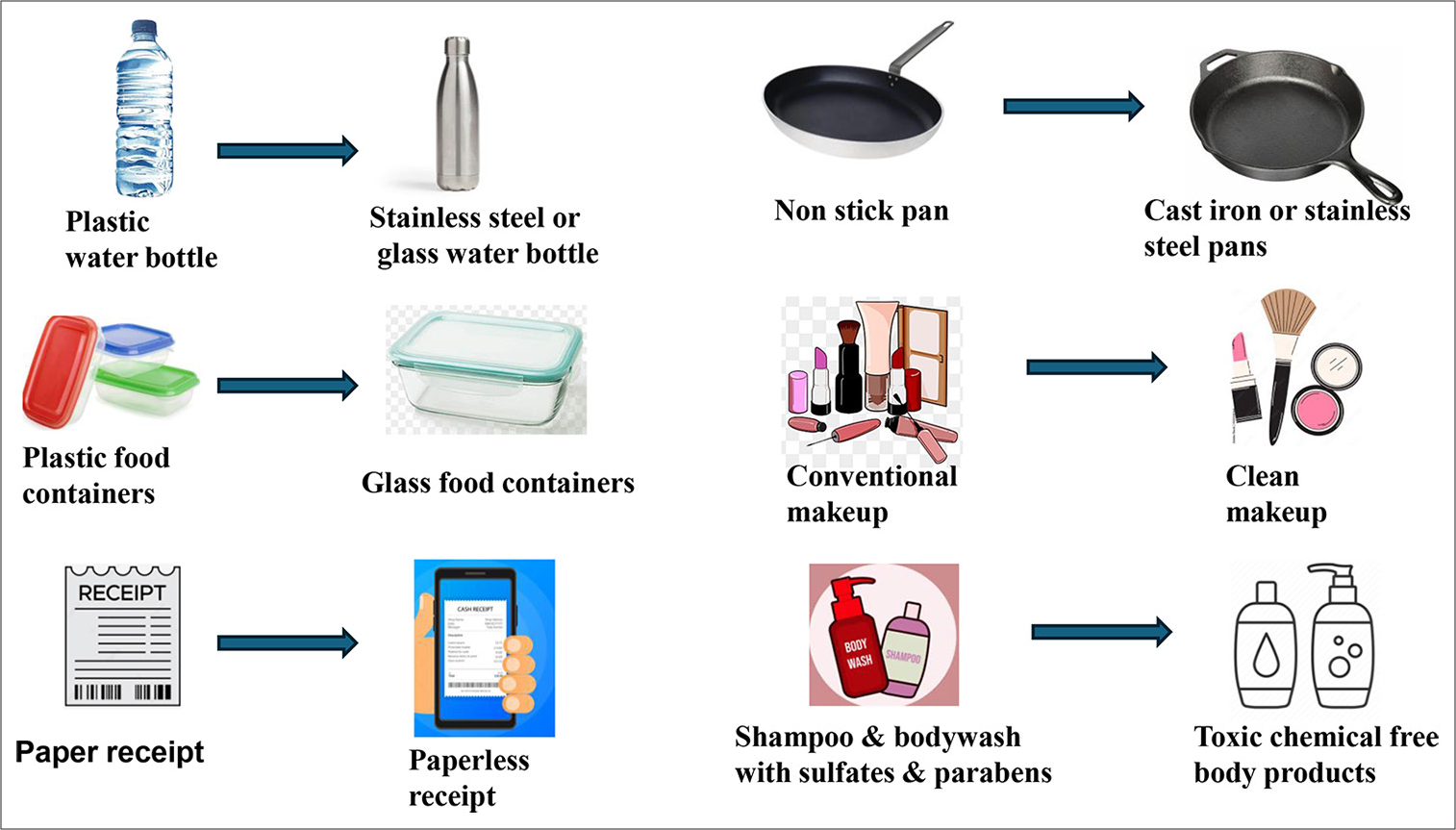
- Easy endocrine disruptor swaps in daily life.
Strategies for reducing EDC exposure at regulatory level:
Strengthening premarketing studies and regulations
Promoting research for safer alternatives
Measures to decrease production of EDCs and entry into ecosystem
Initiatives for raising public awareness
Strong legislative interventions for mitigating exposure of the general public.
The unfortunate fact remains that EDCs are pervasive and cannot be completely removed or avoided. However, individuals and societies can play a crucial role in minimizing EDC exposure and its associated health risks by adopting these practices and advocating for stricter regulations.
CONCLUSION
EDCs are widespread and present in everyday items like plastics, food packaging, personal care products, and even water. Their harmful effects can extend across generations, increasing the risk of obesity, T2D, and other chronic conditions. While small changes in daily lifestyle, such as reducing the use of plastics, choosing organic products, and reading product labels, can help reduce exposure at an individual level, a holistic solution requires steps to be taken at governmental level. Regulatory bodies and lawmakers must implement stricter guidelines and promote safer alternatives to mitigate the long-term impact of EDCs. Hence, protecting the current and future generations from these chemicals demands collective efforts from both individuals and policymakers.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Scientific Opinion on the Hazard Assessment of Endocrine Disruptors: Scientific Criteria for Identification of Endocrine Disruptors and Appropriateness of Existing Test Methods for Assessing Effects Mediated by These Substances on Human Health and the Environment. EFSA J. 2013;11:3132.
- [CrossRef] [Google Scholar]
- Scientific Issues Relevant to Setting Regulatory Criteria to Identify Endocrine-Disrupting Substances in the European Union. Environ Health Perspect. 2016;124:1497-503.
- [CrossRef] [PubMed] [Google Scholar]
- Adenocarcinoma of the Vagina: Association of Maternal Stilbestrol Therapy with Tumor Appearance in Young Women. N Engl J Med. 1971;284:878-81.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental Effects of Endocrine-disrupting Chemicals in Wildlife and Humans. Environ Health Perspect. 1993;101:378-84.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating Levels of Bisphenol A and Phthalates are Related to Carotid Atherosclerosis in the Elderly. Atherosclerosis. 2011;218:207-13.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA. 2008;300:1303-10.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating Levels of Persistent Organic Pollutants (POPs) and Carotid Atherosclerosis in the Elderly. Environ Health Perspect. 2012;120:38-43.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting Chemicals: Associated Disorders and Mechanisms of Action. J Environ Public Health. 2012;2012:713696.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal Phenol and Phthalate Exposures and Birth Outcomes. Environ Health Perspect. 2008;116:1092-7.
- [CrossRef] [PubMed] [Google Scholar]
- Birth Weight and Prenatal Exposure to Polychlorinated Biphenyls (PCBs) and Dichlorodiphenyldichloroethylene (DDE): A Meta-analysis within 12 European Birth Cohorts. Environ Health Perspect. 2012;120:162-70.
- [CrossRef] [PubMed] [Google Scholar]
- Perfluoroalkyl and Polyfluoroalkyl Substances and Human Fetal Growth: A Systematic Review. Crit Rev Toxicol. 2015;45:53-67.
- [CrossRef] [PubMed] [Google Scholar]
- Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J Clin Endocrinol Metab. 2015;100:E1394-403.
- [CrossRef] [PubMed] [Google Scholar]
- Heavy Metal Toxicity and the Environment. Mol Clin Environ Toxicol Environ Toxicol. 2012;3:133-64.
- [CrossRef] [PubMed] [Google Scholar]
- EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-disrupting Chemicals. Endocr Rev. 2015;36:E1-150.
- [CrossRef] [PubMed] [Google Scholar]
- Food Components and Contaminants as (anti) Androgenic Molecules. Genes Nutr. 2017;12:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Wildlife as Models for the Study of How Mixtures, Low Doses, and the Embryonic Environment Modulate the Action of Endocrine-disrupting Chemicals. Pure Appl Chem. 2003;75:2305-20.
- [CrossRef] [Google Scholar]
- Perand Polyfluoroalkyl Substances as Persistent Pollutants with Metabolic and Endocrine-disrupting Impacts. Trends Endocrinol Metab. 2024;23:S1043-2760(24)00202-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu Rev Physiol. 2011;73:135-62.
- [CrossRef] [PubMed] [Google Scholar]
- Synthetic Chemicals and Cardiometabolic Health Across the Life Course among Vulnerable Populations: A Review of the Literature from 2018 to 2019. Curr Environ Health Rep. 2020;7:30-47.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol A and Human Health: A Review of the Literature. Reprod Toxicol. 2013;42:132-55.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30:293-342.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary Bisphenol A Concentration and Angiography-defined Coronary Artery Stenosis. PLoS One. 2012;7:e43378.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. J Cancer Prev. 2015;20:12.
- [CrossRef] [PubMed] [Google Scholar]
- Obesogens: An Emerging Threat to Public Health. Am J Obstet Gynecol. 2016;214:559-65.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol A Exposure During Pregnancy Disrupts Glucose Homeostasis in Mothers and Adult Male Offspring. Environ Health Perspect. 2010;118:1243-50.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting Chemicals, Risk of Type 2 Diabetes, and Diabetes-related Metabolic Traits: A Systematic Review and Meta-analysis. J Diabetes. 2016;8:516-32.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine Disrupters as Obesogens. Mol Cell Endocrinol. 2009;304:19-29.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental Obesogens: Mechanisms and Controversies. Annu Rev Pharmacol Toxicol. 2019;59:89-106.
- [CrossRef] [PubMed] [Google Scholar]
- Minireview: The Case for Obesogens. Mol Endocrinol. 2009;23:1127-34.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac Aging in Mice and Humans: The Role of Mitochondrial Oxidative Stress. Trends Cardiovasc Med. 2009;19:213-20.
- [CrossRef] [PubMed] [Google Scholar]
- Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PloS One. 2013;8:e55387.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal Cadmium Exposure Reduces Placental Zinc Transport and Induces Fetal Growth Restriction in Mice. Reprod Toxicol. 2016;63:174-82.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol A Affects Androgen Receptor Function via Multiple Mechanisms. Chem Biol Interact. 2013;203:556-64.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting Chemicals and Reproductive Health. J Midwifery Womens Health. 2016;61:442-55.
- [CrossRef] [PubMed] [Google Scholar]
- New Modes of Action for Endocrine-disrupting Chemicals. Mol Endocrinol. 2006;20:475-82.
- [CrossRef] [PubMed] [Google Scholar]
- Lead Exposure and Cardiovascular Disease-A Systematic Review. Environ Health Perspect. 2007;115:472-82.
- [CrossRef] [PubMed] [Google Scholar]
- Low-level Lead Exposure and Mortality in US Adults: A Population-based Cohort Study. Lancet Public Health. 2018;3:e177-84.
- [CrossRef] [PubMed] [Google Scholar]
- Cadmium Exposure and All-cause and Cardiovascular Mortality in the US General Population. Environ Health Perspect. 2012;120:1017-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cadmium Exposure is Accompanied by Increased Prevalence and Future Growth of Atherosclerotic Plaques in 64-year-old Women. J Intern Med. 2012;272:601-10.
- [CrossRef] [PubMed] [Google Scholar]
- Cadmium is a Novel and Independent Risk Factor for Early Atherosclerosis Mechanisms and in vivo Relevance. Arterioscler Thromb Vasc Biol. 2009;29:1392-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cadmium and Cardiovascular Diseases: Cell Biology, Pathophysiology, and Epidemiological Relevance. Biometals. 2010;23:811-22.
- [CrossRef] [PubMed] [Google Scholar]
- Hormones and Endocrine-disrupting Chemicals: Low-dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012;33:378-455.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Bisphenol A on Cardiovascular Disease: An Epidemiological Study Using National Health and Nutrition Examination Survey 2003-2016 and Meta-analysis. Sci Total Environ. 2021;763:142941.
- [CrossRef] [PubMed] [Google Scholar]
- Dioxin Exposure and Insulin Resistance in Taiwanese Living Near a Highly Contaminated Area. Epidemiology. 2010;21:56-61.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary Bisphenol A (BPA) Concentration Associates with Obesity and Insulin Resistance. J Clin Endocrinol Metab. 2012;97:E223-7.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolism of Phthalates in Humans. Mol Nutr Food Res. 2007;51:899-911.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Occupational Exposures with Cardiovascular Disease among US Hispanics/Latinos. Heart. 2019;105:439-48.
- [CrossRef] [PubMed] [Google Scholar]
- Concentrations of Urinary Phthalate Metabolites are Associated with Increased Waist Circumference and Insulin Resistance in Adult US Males. Environ Health Perspect. 2007;115:876-82.
- [CrossRef] [PubMed] [Google Scholar]
- Transcriptomics and Metabonomics Analyses of Maternal DEHP Exposure on Male Offspring. Environ Sci Pollut Res. 2018;25:26322-9.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal Diethylhexyl Phthalate Exposure Affects Adiposity and Insulin Tolerance in Offspring in a PCNA-dependent Manner. Environ Res. 2017;159:588-94.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal Low-dose DEHP Exposure Induces Metabolic Adaptation and Obesity: Role of Hepatic Thiamine Metabolism. J Hazard Mater. 2020;385:121534.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the Association between Persistent Organic Pollutants (POPs) and Diabetes in Epidemiological Studies: A National Toxicology Program Workshop Review. Environ Health Perspect. 2013;121:774-83.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal Exposure to di-(2-ethylhexyl) Phthalate Exposure Deregulates Blood Pressure, Adiposity, Cholesterol Metabolism and Social Interaction in Mouse Offspring. Arch Toxicol. 2016;90:1211-24.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Concentrations of Persistent Organic Pollutants and Prediabetes and Diabetes in the General Population of Catalonia. Environ Sci Technol. 2012;46:7799-810.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating Levels of Persistent Organic Pollutants in Relation to Visceral and Subcutaneous Adipose Tissue by Abdominal MRI. Obesity (Silver Spring). 2013;21:413-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ Health Perspect. 2012;120:1658-70.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid Intima-media Thickness and Plasma Asymmetric Dimethylarginine in Mexican Children Exposed to Inorganic Arsenic. Environ Health Perspect. 2013;121:1090-6.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental Arsenic Exposure and Serum Matrix Metalloproteinase-9. J Expo Sci Environ Epidemiol. 2013;23:163-9.
- [CrossRef] [PubMed] [Google Scholar]
- Increases in Oxidized Low-density Lipoprotein and Other Inflammatory and Adhesion Molecules with a Concomitant Decrease in High-density Lipoprotein in the Individuals Exposed to Arsenic in Bangladesh. Toxicol Sci. 2013;135:17-25.
- [CrossRef] [PubMed] [Google Scholar]
- Air Pollution and Incidence of Hypertension and Diabetes Mellitus in Black Women Living in Los Angeles. Circulation. 2012;125:767-72.
- [CrossRef] [PubMed] [Google Scholar]
- Exposures to Particulate Matter and Polycyclic Aromatic Hydrocarbons and Oxidative Stress in Schoolchildren. Environ Health Perspect. 2010;118:579-83.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular Mortality and Long-term Exposure to Particulate Air Pollution: Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation. 2004;109:71-7.
- [CrossRef] [PubMed] [Google Scholar]
- Particulate Matter from Tobacco Versus Diesel Car Exhaust: An Educational Perspective. Tob Control. 2004;13:219-21.
- [CrossRef] [PubMed] [Google Scholar]
- GSTM1, GSTT1, and GSTP1 Polymorphisms and Associations between Air Pollutants and Markers of Insulin Resistance in Elderly Koreans. Environ Health Perspect. 2012;120:1378-84.
- [CrossRef] [PubMed] [Google Scholar]
- Inhalation of Hazardous Air Pollutants from Environmental Tobacco Smoke in US Residences. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S71-7.
- [CrossRef] [PubMed] [Google Scholar]
- Parma Consensus Statement on Metabolic Disruptors. Environ Health. 2015;14:54.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Urinary Phthalate Metabolite Concentrations with Body Mass Index and Waist Circumference: A Cross-sectional Study of NHANES Data, 1999-2002. Environ Health. 2008;7:27.
- [CrossRef] [PubMed] [Google Scholar]
- Perfluoroalkyl Substances and Changes in Body Weight and Resting Metabolic Rate in Response to Weight-loss Diets: A Prospective Study. PLoS Med. 2018;15:e1002502.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Urinary Bisphenol A Concentration and Obesity Prevalence in Children and Adolescents. JAMA. 2012;308:1113-21.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ Health Perspect. 2018;126:37001.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting Chemicals and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis. Environ Health. 2022;21:53.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine Disruptors and Neonatal Anthropometry, NICHD Fetal Growth Studies-Singletons. Environ Int. 2018;119:515-26.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy Urinary Phthalate Metabolite Concentrations and Gestational Diabetes Risk Factors. Environ Int. 2016;96:118-26.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal Exposure to Ambient Pesticides and Preterm Birth and Term Low Birthweight in Agricultural Regions of California. Toxics. 2018;6:41.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between Exposure to Perfluoroalkyl Substances and Birth Outcomes: A Meta-analysis. Chemosphere. 2022;291:132909.
- [CrossRef] [PubMed] [Google Scholar]
- Joint Impact of Phthalate Exposure and Stressful Life Events in Pregnancy On Preterm Birth. Environ Int. 2019;133:105254.
- [CrossRef] [PubMed] [Google Scholar]
- Associations of Perfluoroalkyl Substances (PFAS) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK) Environ Health Perspect. 2015;123:1317-24.
- [CrossRef] [PubMed] [Google Scholar]
- Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function. Environ Health Perspect. 2017;125:481-7.
- [CrossRef] [PubMed] [Google Scholar]
- A Birth Cohort Study to Investigate the Association between Prenatal Phthalate and Bisphenol A Exposures and Fetal Markers of Metabolic Dysfunction. Environ Health. 2014;13:84.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal Urinary Bisphenol A Concentration during Midterm Pregnancy and Children's Blood Pressure at Age 4. Hypertension. 2017;69:367-74.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary Bisphenol A Concentrations and Adiposity Measures at Age 7 Years in a Prospective Birth Cohort. Chemosphere. 2020;251:126340.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating Burden and Disease Costs of Exposure to Endocrine-disrupting Chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1245-55.
- [CrossRef] [PubMed] [Google Scholar]
- Integrative Pediatrics: Art, Science, and Clinical Application United Kingdom: Routledge; 2017.
- [CrossRef] [Google Scholar]
- Effects of Environmental Endocrine-Disrupting Chemicals on Female Reproductive Health. Adv Exp Med Biol. 2021;1300:205-29.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental Endocrine Disruptors: Effects on the Human Male Reproductive System. Rev Endocr Metab Disord. 2015;16:341-57.
- [CrossRef] [PubMed] [Google Scholar]
- Organic Diets Significantly Lower Children's Dietary Exposure to Organophosphorus Pesticides. Environ Health Perspect. 2006;114:260-3.
- [CrossRef] [PubMed] [Google Scholar]
- Food Additives and Child Health. Pediatrics. 2018;142:e20181410.
- [CrossRef] [PubMed] [Google Scholar]
- Perfluorinated Compounds, Polychlorinated Biphenyls, and Organochlorine Pesticide Contamination in Composite Food Samples from Dallas, Texas, USA. Environ Health Perspect. 2010;118:796-802.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to Endocrine Disrupting Chemicals from Beverage Packaging Materials and Risk Assessment for Consumers. J Hazard Mater. 2024;465:133314.
- [CrossRef] [PubMed] [Google Scholar]
- An Overview of the Uses of Per-and Polyfluoroalkyl Substances (PFAS) Environ Sci Process Impacts. 2020;22:2345-73.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental Oestrogens, Cosmetics and Breast Cancer. Best Pract Res Clin Endocrinol Metab. 2006;20:121-43.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Endocrine Disruptor Pesticides: A Review. Int J Environ Res Public Health. 2011;8:2265-303.
- [CrossRef] [PubMed] [Google Scholar]
- Indoor Air Pollutants in Office Environments: Assessment of Comfort, Health, and Performance. Int J Hyg Environ Health. 2013;216:371-94.
- [CrossRef] [PubMed] [Google Scholar]







