Translate this page into:
COVID-19 Impact on Female Patients at Tertiary Care Hospital – A Retrospective Study
*Corresponding author: Dikshant Jain, Department of Cardiology, Safdarjung Hospital, New Delhi, India. dikshant.jain@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta P, Kisku N, Agarwal T, Jain D. COVID-19 Impact on Female Patients at Tertiary Care Hospital – A Retrospective Study. Indian J Cardiovasc Dis Women. 2025;10:23-9. doi: 10.25259/IJCDW_50_2023
Abstract
Objectives:
Corona virus disease-19 (COVID-19) has impacted worldwide leading to a high mortality rate. It has been observed that male mortality rate is higher than females due to which it is crucial to systematically study gender differences in disease manifestations. Our study evaluated the association between mortality in COVID-19-positive cases and gender.
Materials and Methods:
This retrospective study was conducted in the cardiology department at a tertiary care hospital which included 699 patients with a confirmed positive diagnosis of COVID-19 by reverse transcription polymerase chain reaction and >18 years of age. Data from the medical records of the department were collected during an 8-month period (July 2020–February 2021).
Results:
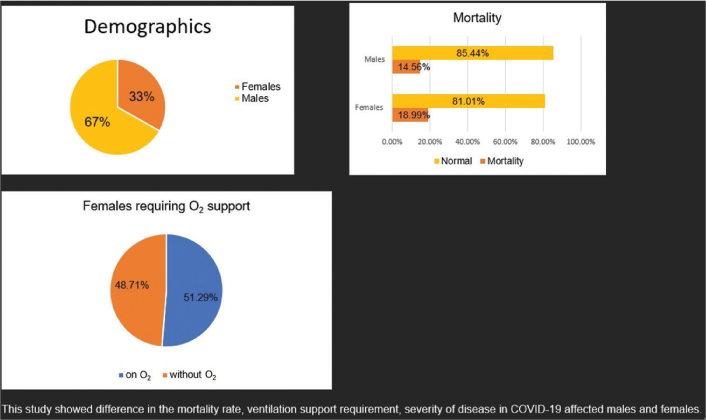
Of the 699 patients enrolled in the study within 8 months, 232 (33.1%) were female and 467 (66.7%) were male. Female mortality accounted for 18.99% and male mortality 14.56%. Comparing the severity of the disease, 36% of females showed more severe disease presentation. In the course of the disease with treatment, 51.29% of females required oxygen support.
Conclusion:
This study showed differences in the mortality rate, ventilation support requirement, and severity of disease in COVID-19 affected males and females.
Keywords
Coronavirus disease-19
Female
Severity of illness
ABSTRACT IMAGE
INTRODUCTION
Globally, coronavirus disease-19 (COVID-19) cases have crossed 70 million, with about 2 million deaths before the end of the year 2021.[1] Male and female mortality rates appear to be similar, although male mortality rates are higher.[2] As many nations enter a second or third wave, it will be crucial to systematically study gender differences in disease manifestations.
Among COVID-19 patients in Europe, only men accounted for 70% of admissions to intensive care units (ICU) and 57% of fatalities.[3] The study by Peckham et al.[4] found that male patients tend to require intensive treatment unit admission almost 3 times as often (odds ratio [OR] = 2.84).[4] A female’s powerful immune response could explain the difference. The impact of the COVID-19 course, however, may be unique and divergent in females due to some diseases common to women such as pregnancy-related complications and ovarian cancer.[5] This study aims to evaluate the association between morbidity and mortality in COVID-19-positive cases and gender. It also reports on the severity of COVID-19 cases based on the number of days spent in the hospital, days in the ICU, and requirements for assisted ventilation based on the experience of a tertiary care hospital for the literature database.
MATERIALS AND METHODS
Study design
This was a retrospective study conducted from July 2020 to February 2021 in the cardiology department at a tertiary care hospital. The study included 699 patients. Ethical clearance was obtained by the ethical committee of the institute.
Inclusion and exclusion criteria
The study population included 699 patients all of whom were confirmed COVID-19 using reverse transcription polymerase chain reaction kits in the hospital laboratory. The age of inclusion was taken as more than 18 years of age. Male patients were taken as controls and female patients were taken as cases. Since this was an observation study, no intervention was planned. The total duration of the patient’s stay was noted and the course of the patient’s stay was observed. Patient comorbidities were noted. All the variables were compared in both the groups including morbidity and mortality. Outcomes of all the patients were noted which included discharge from hospital, prolonged stay, and patient needing ventilator support and mortality.
Data collection
In accordance with [Annexure 1], all data for the retrospective cross-sectional survey of COVID-19 cases enrolled in the study were recorded in our electronic database. Details about demographic variables (age and gender), date of symptom onset, any and all comorbidities, severity according to the guidelines on admission, death/discharge, ICU admission, ICU stay duration, total hospital stay, requirement of oxygen, and requirement of respiratory-assisted devices were collected.
Statistical analysis
The collected data were analyzed statistically using the Mann– Whitney U-test, Chi-square test, and Univariate and Multivariate Logistic Regression (P < 0.05 significant). Statistical analyses have been conducted using the commercially available software Statistics Package for the Social Sciences in the department.
RESULTS
Of the 699 patients enrolled in the study during the 8 months, 232 (33.1%) were female and 467 (66.7%) were male. Out of the total discharged patients at the end of the treatment course, the mean length of hospital stay was comparable for males and females. Female mortality was 18.99% and male mortality was 14.56%. Comparing the severity of the disease, 36% of females showed more severe disease presentation. In the course of the disease with treatment, 51.29% of females required oxygen support. In both groups, ventilator requirements were similar. In the study, all study participants were diagnosed with at least single or multiple comorbidities, which included diabetes, hypertension, thyroid disorders, tuberculosis, lower respiratory tract infection, asthma, and chronic kidney disease [Table 1]. Mortality rates were higher in males than females, 60.71% compared to 39.29% [Table 2].
| Parameter | Female (n=232) |
Male (n=467) |
Overall (n=699) |
|---|---|---|---|
| Age | |||
| N | 232 | 465 | 697 |
| Mean | 47.56 | 44.00 | 45.19 |
| Standard deviation | 18.58 | 16.42 | 17.24 |
| Minimum | 18.00 | 18.00 | 18.00 |
| Maximum | 97.00 | 90.00 | 97.00 |
| Duration of stay (days) | |||
| N | 221 | 453 | 674 |
| Mean | 8.91 | 8.69 | 8.76 |
| Standard deviation | 5.59 | 5.12 | 5.28 |
| Minimum | 0.00 | 0.00 | 0.00 |
| Maximum | 26.00 | 26.00 | 26.00 |
| Mortality, n(%) | |||
| Discharge from hospital | 188 (81.03) | 399 (85.44) | 587 (83.98) |
| Death | 44 (18.97) | 68 (14.56) | 112 (16.02) |
| Severity, n(%) | |||
| Mild | 97 (42.00) | 231 (49.46) | 328 (46.93) |
| Moderate | 51 (22.00) | 94 (20.13) | 145 (20.74) |
| Severe | 84 (36.00) | 142 (30.41) | 226 (32.33) |
| Oxygen requirement, n(%) | |||
| No | 113 (48.71) | 257 (55.03) | 370 (52.93) |
| Yes | 119 (51.29) | 210 (44.97) | 329 (47.07) |
| Ventilator, n(%) | |||
| No | 224 (96.55) | 456 (97.64) | 680 (97.42) |
| Yes | 7 (3.02) | 11 (2.36) | 18 (2.58) |
| Comorbidity condition, n(%) | |||
| Anyone comorbidity | |||
| HTN | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| Asthma | 1 (0.43) | 1 (0.21) | 2 (0.29) |
| Bone TB | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| BPH | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| CAD | 2 (0.86) | 2 (0.43) | 4 (0.57) |
| Depression | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| DM | 14 (6.03) | 34 (7.28) | 48 (6.87) |
| COPD | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| Dyspnea | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| Enteric fever | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| HIV+ | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| HTN | 24 (10.34) | 31 (6.64) | 55 (7.87) |
| Sepsis | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| TB | 4 (1.72) | 9 (1.93) | 13 (1.86) |
| Thyroid disease | 3 (1.29) | 2 (0.43) | 5 (0.72) |
| Two comorbidity conditions | |||
| COPD/DM | 1 (0.43) | 1 (0.21) | 2 (0.29) |
| DM/CKD | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| DM/HTN | 28 (12.07) | 2 (0.43) | 5 (0.72) |
| DM/LRTI | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| DM/TB | 0 (0.00) | 3 (0.64) | 3 (0.43) |
| DM/Typhoid | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| HTN/CAD | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| HTN/COPD | 2 (0.86) | 3 (0.64) | 5 (0.72) |
| HTN/HCV | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| HTN/TB | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| Hypothyroidism/HTN | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| More than two comorbidity | |||
| HTN/AF/CKD | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| HTN/DM/Asthma | 0 (0.00) | 2 (0.43) | 2 (0.29) |
| HTN/DM/Asthma/Jaundice | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| HTN/DM/CAD | 2 (0.86) | 2 (0.43) | 4 (0.57) |
| HTN/DM/CAD | 0 (0.00) | 1 (0.21) | 1 (0.14) |
| HTN/DM/COPD | 0 (0.00) | 3 (0.64) | 3 (0.43) |
| HTN/DM/Hypothyroidism | 1 (0.43) | 0 (0.00) | 1 (0.14) |
| HTN/DM/TB | 4 (1.72) | 6 (1.28) | 10 (1.43) |
| HTN/DM/TB/Asthma | 2 (0.86) | 4 (0.86) | 6 (0.86) |
TB: Tuberculosis, BPH: Benign prostatic hyperplasia, CAD: Coronary artery disease, COPD: Chronic obstructive pulmonary disease, DM: Diabetes mellitus, HIV: Human immunodeficiency virus, HTN: Hypertension, CKD: Chronic kidney disease, HCV: Hepatitis C virus, AF: Atrial fibrillation, LRTI: Lower respiratory tract infections, n: Number of patients
| Parameter | Result-mortality | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Yes (n=112) (%) | No (n=587) (%) | |||
| Male | 68 (60.71) | 399 (67.97) | 0.8932 (0.644, 1.2388) | 0.5541 |
| Female | 44 (39.29) | 188 (32.03) | 1.266 (0.8343, 1.8035) | 0.3109 |
OR: Odds ratio, CI: Confidence interval, n: Number of patients
At an interval of 7 days, the severity of COVID-19 was evaluated based on gender during their hospital stay. During the 1st week after admission, 27.5% of male patients experienced severe COVID-19, compared to 16.23% of female patients [Table 3]. Males reported more severe symptoms than females also during the 2nd week of hospitalization. The odds of severe disease among males and females were compared (OR = 0.76 in males and OR = 0.72 in females) [Table 4].
| Parameter | Result-hospital stay of ≤7 days | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Yes (n=265) (%) | No (n=409) (%) | |||
| Male | ||||
| Mild | 62 (23.40) | 165 (40.34) | 0.5799 (0.4168, 0.807) | 0.0014 |
| Moderate | 44 (16.60) | 46 (11.25) | 1.4763 (0.9496, 2.2951) | 0.0871 |
| Severe | 73 (27.55) | 63 (15.40) | 1.7884 (1.2343, 2.5912) | 0.0023 |
| Female | ||||
| Mild | 25 (9.43) | 70 (17.11) | 0.5512 (0.3404, 0.8927) | 0.0172 |
| Moderate | 18 (6.79) | 28 (6.85) | 0.9922 (0.538, 1.8297) | 1.0000 |
| Severe | 43 (16.23) | 37 (9.05) | 1.7937 (1.1257, 2.8581) | 0.0159 |
OR: Odds ratio, CI: Confidence interval, COVID-19: Coronavirus disease-19, n: Number of patients
| Parameter | Result-hospital stay of 8–14 days | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Yes (n=332) (%) | No (n=342) (%) | |||
| Male | ||||
| Mild | 130 (39.16) | 97 (28.36) | 1.3806 (1.0194, 1.8696) | 0.03842 |
| Moderate | 37 (11.45) | 53 (15.50) | 0.7191 (0.4603, 1.1236) | 0.1776 |
| Severe | 58 (17.47) | 78 (22.81) | 0.766 (0.5281, 1.111) | 0.1877 |
| Female | ||||
| Mild | 50 (15.06) | 45 (13.16) | 1.1446 (0.7445, 1.7597) | 0.5842 |
| Moderate | 24 (7.23) | 22 (6.43) | 1.1238 (0.618, 2.0434) | 0.7615 |
| Severe | 33 (9.94) | 47 (13.74) | 0.7233 (0.452, 1.1573) | 0.1938 |
OR: Odds ratio, CI: Confidence interval, COVID-19: Coronavirus disease-19, n: Number of patients
Table 5 shows the association between COVID-19 symptom severity and hospital stay duration of 15–21 days for male and female patients. It uses Odds ratio (OR) and the P-value to measure the strength and significance of the association. A lower OR means a lower chance of staying in the hospital for 15– 21 days, while a higher P-value means a higher chance of getting the result by random chance. For males with severe symptoms, there is a significant association between symptom severity and longer hospital stays (P = 0.0122). This means that males with severe symptoms are more likely to stay in the hospital for 15–21 days than males with mild or moderate symptoms. The table also shows that for females, there is no significant association between symptom severity and longer hospital stays. This means that females with mild, moderate, or severe symptoms have similar chances of staying in the hospital for 15–21 days. Table 6 compares the morbidity and mortality data among COVID-19 patients, based on their gender, symptom severity, and hospital stay duration of more than 21 days. It uses OR and the P-value to measure the association between these variables. A lower OR means a lower chance of staying in the hospital for more than 21 days, while a lower P-value means a higher statistical significance of the association. Females with mild symptoms have a significantly higher chance of staying in the hospital for more than 21 days than any other group. The OR for females with mild symptoms is 3.3512, which is the highest among all categories, and P = 0.0095, which is the lowest among all categories. Males with severe symptoms have a significantly lower chance of staying in the hospital for more than 21 days than males with mild or moderate symptoms. The OR for males with severe symptoms is 0.2351, which is the lowest among all categories, and P = 0.0122, which is the second lowest among all categories. It does not show any significant association between gender and hospital stay duration, regardless of symptom severity. This means that males and females have similar chances of staying in the hospital for more than 21 days, given their symptom severity.
| Parameter | Result-hospital stay of 15–21 days | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Yes (n=59) (%) | No (n=615) (%) | |||
| Male | ||||
| Mild | 28 (47.46) | 199 (32.36) | 1.4667 (0.91, 2.364) | 0.1199 |
| Moderate | 9 (15.25) | 81 (12.17) | 1.1582 (0.5533, 2.4243) | 0.8444 |
| Severe | 3 (5.08) | 133 (21.63) | 0.2351 (0.0726, 0.7614) | 0.0122 |
| Female | ||||
| Mild | 12 (20.34) | 83 (13.50) | 1.507 (0.7776, 2.9206) | 0.2535 |
| Moderate | 3 (5.08) | 43 (6.99) | 0.7272 (0.2189, 2.4158) | 0.7887 |
| Severe | 4 (6.78) | 76 (12.36) | 0.5486 (0.1938, 1.5527) | 0.2937 |
OR: Odds ratio, CI: Confidence interval, COVID-19: Coronavirus disease-19, n: Number of patients
| Parameter | Result-hospital stay of >21 days | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Yes (n=18) (%) | No (n=656) (%) | |||
| Male | ||||
| Mild | 7 (38.89) | 220 (33.54) | 1.1596 (0.478, 2.8134) | 0.8152 |
| Moderate | 0 (0.00) | 90 (13.72) | NA | NA |
| Severe | 2 (11.11) | 134 (20.43) | 0.5439 (0.1247, 2.3721) | 0.5543 |
| Female | ||||
| Mild | 8 (44.44) | 87 (13.26) | 3.3512 (1.4148, 7.938) | 0.0095 |
| Moderate | 1 (5.56) | 45 (6.86) | 0.8099 (0.1057, 6.205) | 1.0000 |
| Severe | 0 (0.00) | 80 (12.20) | NA | NA |
OR: Odds ratio, CI: Confidence interval, COVID-19: Coronavirus disease-19, NA: Not applicable, n: Number of patients
Furthermore, we compared the morbidity and mortality data among COVID-19 patients, based on their gender and ventilator requirement. Table 7 shows the number and percentage of patients who required a ventilator versus those who did not, along with the OR and P-value for each category. The table shows that ventilator requirement is strongly associated with symptom severity and gender. Ventilator requirement is highest among males with severe symptoms, followed by females with severe symptoms, and lowest among mild and moderate cases for both genders. Males with severe symptoms have a significantly higher risk of requiring a ventilator than any other group. The OR for males with severe symptoms is 3.1722, which means that they are more than 3 times as likely to require a ventilator as the reference group (females with mild symptoms). The P-value for males with severe symptoms is 0.0048, which means that there is a very low probability of getting this result by chance alone. The table also shows that females with severe symptoms have a significantly higher risk of requiring a ventilator than females with mild or moderate symptoms, but not as high as males with severe symptoms. The OR for females with severe symptoms is 2.906, which means that they are almost 3 times as likely to require a ventilator as the reference group. The P-value for females with severe symptoms is 0.0122, which means that there is a low probability of getting this result by chance alone. Mild and moderate cases have similar risks of requiring a ventilator, regardless of their gender. The ORs for mild and moderate cases are close to 1, which means that they have similar odds as the reference group.
| Parameter | Result-ventilator requirement | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Yes (n=18) (%) | No (n=680) (%) | |||
| Male | ||||
| Mild | 0 (0.00) | 231 (33.97) | NA | NA |
| Moderate | 0 (0.00) | 94 (13.82) | NA | NA |
| Severe | 11 (61.11) | 131 (19.26) | 3.1722 (1.4643, 6.8722) | 0.0048 |
| Female | ||||
| Mild | 0 (0.00) | 96 (14.12) | NA | NA |
| Moderate | 1 (5.56) | 50 (7.35) | 0.7556 (0.0988, 5.7764) | 1.0000 |
| Severe | 6 (33.33) | 78 (11.47) | 2.906 (1.1203, 7.5381) | 0.03525 |
OR: Odds ratio, CI: Confidence interval, COVID-19: Coronavirus disease-19, NA: Not applicable, n: Number of patients
DISCUSSION
Based on our experience at a tertiary care hospital for an 8-month period, we report that the male gender is more likely to be affected with severe COVID-19 by a mean age of 45 years. However, both groups reported comparable hospital stays. The mortality rate in female patients was 18.97%, which was slightly higher than the mortality rate in males. The number of severe cases accounted for 32.33% of all cases, with 36% of females and 30.41% of males, suggesting that the severity of the condition was more observed in females. A study conducted by Wu et al. from Hubei Province showed similar results in China and reported the COVID-19 positivity rate of 47.81% among females with a median age of 50.53 years and severe cases in 20.11%.[2] US metropolitan COVID-19 surveillance and outcome registry data analysis showed that the COVID-19 positivity rate was higher in males than in females with OR = 1.20.[4] Another study by Raimondi et al. found that mortality from COVID was 38.1% in men and 26.1% in women.[6]
As observed in the present study, patients with COVID-19 diagnoses often had comorbidities such as hypertension, diabetes, tuberculosis, thyroid disorders, and chronic kidney disease, among others. Coronary artery disease patients were often seen to be affected by COVID-19. There were patients with single or multiple comorbidities, and similar reports have been published by Wu et al. in the Chinese population.[2] Other studies have also yielded similar results.[6-9]
The present retrospective study investigated the higher rates of diabetes and hypertension associated with COVID-19 cases. In severe acute respiratory syndrome coronavirus 2 (SARS-COV2) patients with diabetes and hypertension as comorbidities, Wu et al. reported a higher r isk of acute respiratory distress syndrome (ARDS), (OR – females: 0.94, male: 1.13). However, the correlation between ARDS progression and death in this group of patients was not pursued.[10]
According to Guan et al., patients with diabetes and hypertension comorbidities have approximately 1.58-fold increased mortality risk.[7] There is limited evidence for gender-specific differences in mortality numbers correlated with comorbidities.
Among male patients in hospitals, the mortality rate was 60.71%, a higher rate than that of female patients at 39.29%. Similarly, 11.6% of males experienced in-hospital mortality, which was higher than the 8.3% death rate among females in a study conducted among US metropolitan area residents.[11]
In the hospital, it was observed that male patients were more susceptible to SARS-CoV-2, severity of the illness, length of hospital stays, and need for ventilator support. Numerous studies have shown similar results in Europe, China, and the US.[12,13] Understanding disease severity, progression, and cause of death is essential to disease management and outcome prediction for men and women alike.
Uncertainty surrounds the underlying mechanism of gender predilection in COVID-19. However, gender predilection and COVID-19 course in males might be influenced by higher comorbidity rates, such as diabetes, hypertension, cardiovascular disease, and chronic obstructive pulmonary disease. Females and males have different immune responses. Females tend to be more resistant to pathogens due to their stronger antigenic response to antigens and vaccines.[12,14]
Angiotensin-converting enzyme 2 receptors and transmembrane protease with gender differences are essential for viral entry.[15] Accordingly, SARS-CoV-2 patients have higher levels of inflammatory cytokines and chemokines, and, at baseline, female patients have more T-cells activation than male patients. An insufficient T-cell response is associated with a worse outcome, and recently, it was revealed that Kynurenic acid (KYNA) is derived from one of the tryptophan metabolism pathways, kynurenine. KYNA is associated with the immune system and inflammation. KYNA is particularly noteworthy due to its anti-inflammatory and immunosuppressive properties. Due to these characteristics, KYNA could be considered a “double-edged sword.” In addition to resolving inflammation, metabolites can be a part of establishing an immunosuppressive environment, resulting in a gender-specific immune response.[16] Due to the fact that gender has been identified as a disease modifier, it is imperative to study the biological pathways responsible for gender-specific disease etiology.
Limitations
The study has a few limitations. The patients included in the study were the ones those who presented to our hospital, rather than choosing from the community – the hospital bias. The study included a larger proportion of males. The total number of study participants was 699, with insufficient sample size to extrapolate to the general population. The mechanism of action for COVID-19 has been inadequately understood. Our understanding has been lacking on the variety of individual responses to COVID-19. Even if we equate all the variables amongst the females, why COVID-19 leads to severe disease in few and mild disease in others is beyond the scope of our study.
CONCLUSION
This study highlights the difference in COVID-19 disease presentation, severity, ventilation support, and mortality between males and females. Females presented with more severe disease at the onset which translated to more oxygen requirement and a slightly increased rate of ventilator requirement. However, males had a higher mortality overall. Men and women have different responses to environmental insults due to hormonal differences, which could contribute to gender-specific differences. Further, research understanding the molecular basis of this difference and the difference in responses among males and females and even among individuals of the same gender needs to be explored.
Ethical approval
The research/study was approved by the Institutional Ethics Committee, S.No. IEC/VMMC/SJH/Trial/2022-02/CC-240.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Audio summary available at
Annexure available on
Financial support and sponsorship: Nil.
References
- Global Perspective of COVID-19 Epidemiology for a Full-cycle Pandemic. Eur J Clin Invest. 2020;50:e13423.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Comorbidities and the Risk of Death in Patients with COVID-19: Sex Specific Difference. medRxiv [Preprint]; 2020
- [CrossRef] [Google Scholar]
- Consensus Scientific Statement on Advisory Working Guidelines and Recommendations for the Female Population in COVID-19 Era by WINCARS. Indian J Cardiovasc Dis Women WINCARS. 2020;5:175-94.
- [CrossRef] [Google Scholar]
- Male Sex Identified by Global COVID-19 Meta-analysis as a Risk Factor for Death and ITU Admission. Nat Commun. 2020;11:6317.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of COVID-19 Pandemic on Ovarian Cancer Management: Adjusting to the New Normal. Cancer Manag Res. 2020;13:359-66.
- [CrossRef] [PubMed] [Google Scholar]
- Covid-19 and Gender: Lower Rate but Same Mortality of Severe Disease in Women-An Observational Study. BMC Pulm Med. 2021;21:96.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity and Its Impact on 1590 Patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020;55:2000547.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Characteristics of 113 Deceased Patients with COVID 2019: Retrospective Study. BMJ. 2020;368:m1091.
- [CrossRef] [PubMed] [Google Scholar]
- Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Covid 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-43.
- [CrossRef] [PubMed] [Google Scholar]
- Sex Differences in Susceptibility, Severity, and Outcomes of Coronavirus Disease 2019: Cross-sectional Analysis from a Diverse US Metropolitan Area. PLoS One. 2021;16:e0245556.
- [CrossRef] [PubMed] [Google Scholar]
- Sex Differences in Immune Responses. Nat Rev Immunol. 2016;16:626-38.
- [CrossRef] [PubMed] [Google Scholar]
- Gender and Covid-19 Project. 2020. Available from: https://globalhealth5050.org/covid19 [Last accessed on 2023 Aug 08]
- [Google Scholar]
- The Impact of Sex and Age on T Cell Immunity and Ischemic Stroke Outcomes. Cell Immunol. 2019;345:103960.
- [CrossRef] [PubMed] [Google Scholar]
- TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discov. 2020;10:779-82.
- [CrossRef] [PubMed] [Google Scholar]
- Kynurenic acid Underlies Sex-specific Immune Response to COVID-19. Sci Signal. 2021;14:eabf8483.
- [CrossRef] [PubMed] [Google Scholar]







