Translate this page into:
Complete Atrioventricular Canal Defect with Infective Endocarditis in a Neonate
V.S. Bharathi Lakshmi, MD, DM (Cardiology) Department of Cardiology, Nizam’s Institute of Medical Sciences Punjagutta, Hyderabad India bharathivanaparty@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
This is the case of a new born baby with complete atrioventricular (AV) canal defect with common atrium and vegetations on the common AV, pulmonary, and aortic valves.
Keywords
atrioventricular canal defect
infective endocarditis
neonate
Introduction
A 3-day-old neonate hospitalized with congestive heart failure. Two-dimensional (2D) echocardiogram static images were presented in Fig. 1. What is the full diagnosis?
-
Opinion 1: Atrioventricular (AV) canal defect, ventricular septal defect (VSD), mild mitral regurgitation (MR), and left superior vena cava
-
Contributor: Anything else?
-
Opinion 2: Also, patent ductus arteriosus (PDA).
-
Opinion 3: Ebstein anomaly and hypoplastic right heart syndrome. Single great vessel, that is, aorta.
-
Opinion 4: Complete AV canal defect with large atrial septal defect (ASD) nearly a common atrium with mild MR and PDA with doming of pulmonary valve and poststenotic dilatation of pulmonary artery, pulmonary stenosis, must be there...
-
Opinion 5: Common atrium, AV canal defect, PDA, and MR.
Contributor—Final diagnosis: Congenital heart disease, situs solitus, complete AV canal defect with common atrium and PDA with vegetation on aortic, pulmonary and tricuspid valve. MR with three jets due to cleft mitral valve and another jet due to perforation of anterior mitral leaflet (AML) in just born baby.
Discussion
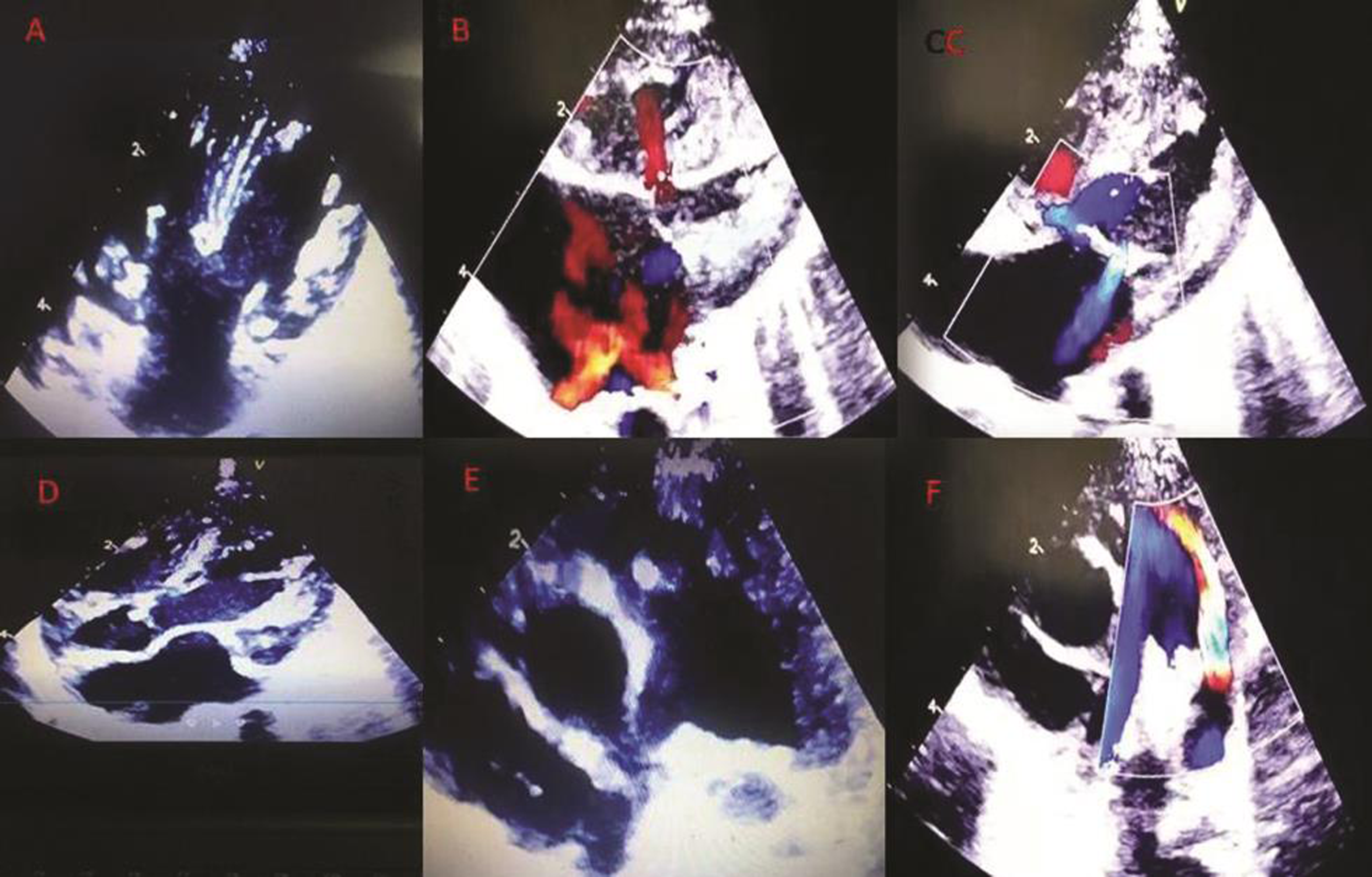
Fig. 1 shows the echocardiographic pictures taken in a neonate. Fig. 1 (a) shows the subcostal four-chamber view which consists of a common atrium, common AV valve, and an inlet VSD. It is a complete AV canal defect, a balanced type. A small mobile mass, a vegetation can be seen on the right side of the AV valve (Fig. 2 (a)). In Fig. 1 (b), the four-chamber color Doppler shows normal pulmonary venous return and left-to-right shunt across the VSD. In Fig. 1 (c), a mild-to-moderate MR is also seen. The cleft in the valve or the perforation could not be clearly appreciated in the pictures. But the contributors have opined that there were three jets of MR due to a cleft in the left mitral valve and a perforation of the AML, which might have been visualized in the motion images. Fig. 1 (d) shows the subcostal five-chamber view which consists of a vegetation on the aortic valve (Fig. 2 (b)). In Fig. 1 (e), the parasternal short axis view shows a vegetation on the pulmonary valve (Fig. 2 (c)). PDA with continuous left-to-right shunt is seen in the color Doppler parasternal short axis view(Fig. 1 (f)).
-
Fig. 1 (a-f) Static 2D echo images.
Fig. 1 (a-f) Static 2D echo images.
-
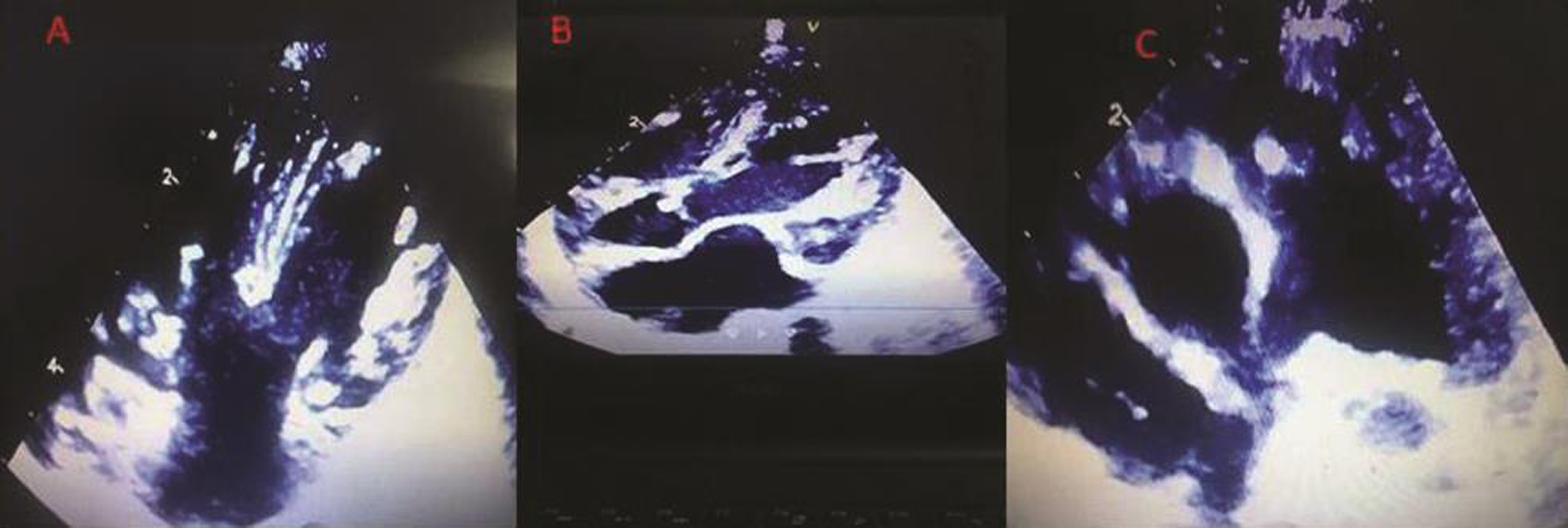
Fig. 2 (a-c) Vegetations on right AV valve, aortic valve and pulmonary valve. Abbreviation: AV, atrioventricular.
Fig. 2 (a-c) Vegetations on right AV valve, aortic valve and pulmonary valve. Abbreviation: AV, atrioventricular.
The complete AV canal defect is characterized by the common AV valve, common annulus, ostium primum ASF, and an inlet VSD.
The common features of all forms of AV canal defects are absence of the AV septum, unwedging of the aortic valve, elongation of the left ventricular outflow tract (LVOT), cleft of the left AV valve, and clockwise rotation of the LV papillary muscles.1
The absence of the AV septum, that is, the insertion of the right and left AV valve components at the same level is echocardiographically best appreciated in the apical four-chamber view which demonstrates the crux of the heart.
The unwedging of the aortic valve, resulting in anterior displacement of the aortic valve is best appreciated in parasternal long axis and subcostal outflow views.
The anterior displacement of the unwedged aortic valve results in an elongated and narrow LVOT, which is described as a goose neck appearance and can be identified in echocardiographic long axis views of the LV.
In the parasternal short-axis view, the LV papillary muscles are seen in the 3 o’clock and 7 o’clock positions instead of the 4 o’clock and 8 o’clock positions due to the counter clockwise rotation 0.2
A cleft of the left AV valve component is a characteristic feature of AV canal defects. In partial AV defects, the cleft in the AML is directed toward the midportion of the ventricular septum. In contrast, in patients with isolated cleft of the mitral valve, the cleft is directed toward the LVOT. In complete AVSD, the common AV valve consists of five leaflets, the superior bridging leaflet, inferior bridging leaflet, left lateral leaflet, right lateral leaflet, and right anterior leaflet. The space between the anterior and posterior bridging leaflets is analogous to the cleft in the anterior mural leaflet in partial AVSD.3 4
Determining balance with echocardiography is important as it forms the basis for deciding on single ventricular versus biventricular surgical pathway.
AVSD can be balanced or unbalanced based on how the AV junction is shared by the ventricles. In balanced AVSD, the AV inlet is more or less equally shared by the two ventricular chambers.5
In unbalanced AVSD, more than half of the AV junction is committed to one of the ventricles which is the dominant ventricle and the other ventricle is typically hypoplastic. Unbalanced AVSD occurs in 10 to 15% of AVSDs. out of which two-thirds are right ventricular (RV) dominant. Unbalanced AVSDs with LV dominance have a hypoplastic RV and are associated with pulmonary stenosis or atresia. Unbalanced AVSDs with RV dominance have a hypoplastic LV and are typically associated with coarctation of aorta and arch anomalies.
Relative size of the ventricles is best appreciated from the apical four-chamber view. This view also allows visualization of malalignment between the atria and ventricular septa in complete AVSD, another clue to an unbalanced AVSD.
Evaluation of the common AV valve morphology is crucial to identify mechanism of regurgitation, commitment to the RV and LV masses, attachments, and existence of possible anomalies such as double orifice left AV valve.
The best view that visualizes the anatomy of the valve is the enface view, obtained from the subcostal window by rotating the transducer clockwise from the four-chamber view.
The subcostal enface view of the AV valves gives an estimate of the proportion of the AV valve area committed to each ventricle. AV valve index: the ratio of area of the AV valve apportioned over each ventricle calculated in this view has been shown to be useful to stratify patients into single or biventricular pathway 0.2.
The Rastelli classification of the common AV valve, based on degree of the bridging of the anterior bridging leaflet and the chordal attachments is also helpful in preoperative assessment.6
The intracardiac mass lesions in this case are most likely vegetations because of the intrinsic mobility and location. They have to be differentiated from intracardiac thrombi as it is an uncommon but severe complication of central venous catheters. The other mass lesions to be differentiated are tumors like rhabdomyomas and fibromas, but there is no evidence of increased incidence of cardiac tumors with congenital heart disease.
2D echo gives a good preoperative assessment of AV canal defect, but sometimes the mechanism of AV regurgitation is difficult to determine. Although there is a lot of improvement in surgical techniques in AVSDs, early and late AV valve regurgitations pose problems. LVOT obstruction may occur in 4 to 12% of cases. Three-dimensional (3D) echocardiography better demonstrates the various relationships between the valve leaflets and septal structures, and it is very helpful in the evaluation of pre- and postoperative valve failures.7
In a multicenter review, 7.3% cases of pediatric infective endocarditis were diagnosed in the first month of life. The increasing use of invasive techniques, often associated with central venous catheters in patient management in the neonatal intensive care unit, have increased the risk of infective endocarditis.8 Neonates often have heavy episodes of bacteremia due to hyperalimentation, vigorous endotracheal suctioning, and trauma to the skin and mucous membranes. The indwelling lines, either for antibiotic usage or alimentation, become sources of infection in the neonates and may cause endocarditis, which frequently involves the right-sided heart structures. It has been estimated that less than one-third of the neonatal endocarditis occurs in the presence of congenital cardiac disease.9 Prematurity is one of the risk factors for infective endocarditis. A recent review showed that 31% of the infants who died of infective endocarditis were premature.10
The most common organisms were staphylococcus aureus, streptococcus, coagulase negative staphylococcus, Gram negative bacilli and fungi esp candida species. The clinical manifestations of infective endocarditis in the neonate are nonspecific, variable and indistinguishable from septicemia or congestive cardiac failure. A new or changing heart murmur may be present. Neurological manifestations like seizures, hemiparesis or apnea may be present. Septic emboli are common, resulting in foci of infection outside the heart (e.g., osteomyelitis, pneumonia or meningitis).11 Arthralgia, arthritis, Janeway lesions, Osler nodes, and Roth spots are not seen.
Improved and widely available imaging technology, particularly echocardiography and increased clinical awareness, has facilitated diagnosis in the neonatal period. Neonatal infective endocarditis still has a high-mortality rate.
The appropriate management in this case would be a complete hemogram, blood culture and sensitivity, and higher appropriate antibiotics based on culture report. PDA closure can be tried with ibuprofen as it is a neonate. Elective surgical correction can be planned later on.
Acknowledgments
This case was submitted by Prof. I.B. Vijayalaxmi.
We thank Dr. IB Vijayalakshmi (Professor, Bangalore Medical College and Research Institute) for contributing with regard to the images. We thank Dr. Asha Mahilmaran (Consultant Cardiologist, Apollo Hospitals, Chennai), Dr. Shagun Aggarwal (Associate Professor, Department of Genetics, Nizam’s Institute of Medical Sciences), and Dr. Gowri A (Consultant Cardiologist, Sreenivasa Hospital, Kadapa) for providing their expert opinions.
Conflicts of Interest
None declared.
References
- Assessment of atrioventricular septal defects by two dimensional echocardiography. Br Heart J. 1982;47(02):109-121.
- [Google Scholar]
- Common Atrioventricular Canal Defects.Echocardiography in Pediatric and Congenital Heart Disease. 2016. p. :259-278. In: et al., eds.
- [Google Scholar]
- Isolated cleft of the mitral valve: clinical spectrum and course. Tex Heart Inst J. 2009;36(06):553-556.
- [Google Scholar]
- Atrioventricular canal ventricular septal defect with cleft mitral valve. Angiographic and echocardiographic features. Pediatr Cardiol. 1982;2(04):289-295.
- [Google Scholar]
- Morphometric analysis of unbalanced common atrioventricular canal using two-dimensional echocardiography. J Am Coll Cardiol. 1996;28(04):1017-1023.
- [Google Scholar]
- Anatomic observations on complete form of persistent common atrioventricular canal with special reference to atrioventricular valves. Mayo Clin Proc. 1966;41(05):296-308.
- [Google Scholar]
- Echo-morphological correlates in patients with atrioventricular septal defect and common atrioventricular junction. Cardiol Young. 2006;16(03):43-51.
- [Google Scholar]
- Characteristics of children hospitalized with infective endocarditis. Circulation. 2009;119(06):865-870.
- [Google Scholar]
- Neonatal endocarditis–neither rare nor fatal. Clin Pediatr (Phila). 1998;37(12):747-748.
- [Google Scholar]
- The changing spectrum of neonatal endocarditis. Clin Perinatol. 1988;15(03):587-608.
- [Google Scholar]







