Translate this page into:
Comparative Study of Physiological Indicators of Cardiovascular Fitness such as Heart Rate Variability, Harvard Step Test, Ankle-brachial Index, and Body mass Index in Rural and Urban Adolescent Girls
*Corresponding author: Vadde Sai Prathyusha, 3rd Year MBBS student, ESIC Medical College, Hyderabad, Telangana, India. saiprathyushav369@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Prathyusha VS, Katravath S, Fatima F, Mudunuru AK, Reddy SS. Comparative study of physiological indicators of cardiovascular fitness such as heart rate variability, Harvard step test, ankle-brachial index, and body mass index in rural and urban adolescent girls. Indian J Cardiovasc Dis Women 2022;7:84-98.
Abstract
Objectives:
Health is defined as a state of complete physical, mental, and social well-being.
Materials and Methods:
Humans have settled in both urban and rural areas, which might have influenced people and their physiology in different ways.
Results:
Cardiovascular fitness as a marker of proper functioning of the whole body is associated with many health-related outcomes, with poor fitness leading to development of cardiovascular diseases (CVD).
Conclusion:
This study aimed to perform a comparative study of the physiological parameters’ indicative of cardiovascular fitness in urban and rural adolescent girls.
Keywords
Ankle–brachial index
Cardiovascular fitness
Heart rate variability
Heart rate variability
Rural
Urban
INTRODUCTION
The human populations have settled in various geographical locations from the beginning of time. There has been a broad division of these locales into urban and rural areas. Urban areas are identified with higher population per unit area, greater activity, and commerce. Rural areas are synonymous with a quieter life, agriculture, etc. Such differences have been proposed to have played a role in changing some of the physiological parameters too in the urban and rural populations. The present study intends to determine whether any significant difference exists between the values of these parameters among the two groups.
The World Health Organization defines health as a state of complete physical, mental, and social well-being.[1] The harmonious balance of this state of the human individual integrated into his/ her environment constitutes health. Health is a dynamic phenomenon that fluctuates frequently and subtly, ranging from optimum well-being to various levels of dysfunction, including death. The factors which influence health lie within the individual and the community they live in (socioeconomic factors), interaction of these factors may be health promoting or deleterious.
Ability and capacity to perform daily routine activities and tasks enthusiastically and effectively are named as physical fitness of a person.[2] Fitness is an important factor which improves the cardiovascular health. Cardiovascular fitness (CVF), as a marker of proper functioning of the whole body, is associated with many health-related outcomes.[3] CVF is known to be associated with body fat,[4,5] arterial compliance,[4,6] and balance in autonomic functions, which when altered beyond physiological range(s) would eventually lead to development of cardiovascular diseases (CVD). Poor fitness is thus a risk factor for developing CVD which are usually manifested at an advanced stage of pathology. As cardiac diseases are the root cause of many deaths globally, early identification and intervention will prevent progression of diseases and development of complications. Cardiovascular system (CVS) complications are more common in females. At present, females of younger age group are also being affected due to sedentary lifestyle, low socioeconomic status, unawareness, etc. Adolescence is a critical time for the preservation or loss of cardiovascular health.[7] Moreover, CVF seems to prevent premature mortality which supports the concept that CVF may exert a protective effect on the cardiovascular system from an early age.[4,8] Usually, knowledge, attitude, and practices (KAP), which depend on various social, cultural, and economic factors, play a significant role in cardiovascular health. Hence, this study intended to know any the differences in the CVF tests in the rural and urban population, which if present can indicate presence of risk factors. In such a scenario, appropriate primordial prevention measures can be applied to preserve health.
REVIEW OF LITERATURE
Most studies focus on the adult health and diseases. Comparatively, fewer studies have been done on adolescents.
In a study done by Ghosh, it was seen that although prevalence of obesity may not be restricted to either urban or rural population/areas, but higher numbers were seen in urban areas/urban populations.[9]
In a study done by Rose-Clarke et al. on adolescent girls in rural Eastern India, it was observed that thinness was prevalent in 14% of younger girls and 6% of older girls (2SD median BMI for age and sex). Girls were stunted in 45% of cases (2SD median height for age and sex).[10]
According to the Contreras et al.,[11] rural low-income preschoolers in the Midwest USA had higher BMI and engaged in more obesity-promoting eating habits, notably emotional overeating, as compared to urban preschoolers. They also did more physical activity than urban toddlers.
Choudhary et al. have observed in their study that the majority of the underweight girls in their study belonged to the rural region, although overall BMI was higher for the rural group compared to the urban group.[12]
Maiti et al. made the same observation in their study with the lower BMI being observed for the rural population, compared to the urban population.[13]
Tummala et al. have proposed that ankle brachial index (ABI) is one of the easier and better indicators to assess the presence of peripheral arterial disease, especially in the rural areas which may have lack of more sophisticated radiological equipment.[14]
de Oliveira Alvim et al. in their study have found lower prevalence of peripheral arterial disease in the rural populations, although its incidence increases with age.[15]
It has been recommended by some authors that post-exercise ABI is a better indicator of CVF, but Gouveri et al. disagree with this. According to them, post-exercise ABI does not increase sensitivity for the detection of PAD.[16]
A study done on Chinese adolescents, aged 12–18 years, from 1991 to 2011 by Zhang, revealed that CVD risk biomarkers increased in Chinese adolescents. Urbanization was not so favorable for girls as for boys, especially for BMI.[17]
According to a study done by McCarthy et al., rural girls showed a higher BMI, than urban girls. The study concluded that rural living was linked to a higher incidence of CVD in Chinese children.[18]
As per Zhao et al., BMI is one of the indicators of obesity, which can predict cardiovascular risk in adolescents.[19]
According to Gutin, physical activity does contribute to the future cardiovascular health and this could be through modulation of autonomic activity on the heart.[10] This study also showed a favorable association between better heart rate variability (HRV) profiles and greater CVF in adolescents.[20]
A study was done by Farah et al. indicate that the lower HRV shows association with clustering of cardiovascular risk factors in adolescent boys.[21]
A study conducted by Tripathy et al. showed no significant urban/rural differences in dietary habits, physical activity, and obesity, although rural females were involved in strenuous activity than urban females.[22]
Jacobs et al.[23] report that rural dwellers have a greater frequency of significant health issues such as raised blood pressure and diabetes and are more likely to indulge in risky behaviors such as drug usage and drinking. According to their study, rural setting is not so conducive for adolescent health because of the lower income and education levels and increased frequency of chronic diseases observed in the rural population.
Aim of the study
The aim of the study was to perform a comparative study of the physiological parameters indicative of CVF in urban and rural adolescent girls
Objectives of the study
The objectives of the study are as follows:
To perform CVF indicator tests in the rural and urban adolescent girls.
To compare the results in the age matched rural and urban girls, both belonging to non-medical background.
MATERIALS AND METHODS
This study was approved by the Institutional Ethics Committee, ESIC Medical College, Sanathnagar, Hyderabad, Telangana. Before the start of the trial, all the details and procedure of the study were explained to all participants in their native language.
Adolescents between the age 12 and 19 years were invited to participate in the study. All participants gave their informed consent. Because we are primarily concerned with adolescent females, parental or guardian consent was also obtained. The consent forms were explained in their local language.
Rural areas were identified as per the standard norms of population density, facilities etc. around the city of Hyderabad. As part of health camps or routine visits by the Community Medicine department, rural areas were visited and adolescent girls were educated about the need of the study and eventually recruited in to the study using simple random sampling method. Healthy adolescent relatives of the patients who visit the hospital regularly and who stay in a rural area were also followed up and recruited in to the study as part of the rural group.
The urban group was selected from the areas of the city of Hyderabad from relatives of patients who visit ESIC Hospital or from acquaintances who are not relatives/friends of the investigators.
A questionnaire was designed to know the level of KAP of the subjects. Internal validation of the questionnaire was done with a Cronbach’s alpha score of 0.7.
Questions were categorized as those which tested their knowledge about health and risk factors for development of disease, their attitude toward them and the day-to-day practices observed by the subjects. In the questionnaire, eight questions were related to knowledge, five on attitude, and 12 questions on practices followed by them. Additional questions were present to get more information about a particular practice, if it was done or practiced by the respondent. Scoring was done for the questions ranging from 5 to 1, with 5 being the best response and 1 being the least response.
For the sake of simplicity and ease of calculation, negative questions were not framed; rather the questions were framed in such a way that the better response was always 5, be it placed in any order in the multiple-choice options. We designed the questions such that a higher total score indicated better KAP by the subject. This was helpful not only in our calculations, but also removed the predictability factor for respondents. They have to take their time in selecting their responses and not just select the first option that they come across.
Sample size
One hundred adolescent girls, 50 rural and 50 urban, were recruited in the study.
Study design
This was a cross-sectional analytical study.
Site of study
This study was conducted at ESIC Medical College, Sanathnagar, Hyderabad.
Duration
The duration of the study was 7 months. Our sample collection was slightly delayed due to the ongoing COVID-19 pandemic leading to hesitancy on part of the subjects/guardians.
Inclusion criteria
All apparently healthy adolescent girls aged 12–19 years were included in the study. The World Health Organization (WHO) defines “Adolescents” as individuals in the 10– 19 years age group.[24]
Exclusion criteria
Girls with known chronic disorders such as hypothyroidism, anemia, metabolic syndrome, with or without on treatment, and girls who are known cases of having cardiovascular disorders or respiratory ailments, endocrinopathies such as hypothyroidism or juvenile diabetes, and any other chronic diseases which may affect the exercise ability, cardiovascular performance, and parameters were excluded from the study. Treatment for any disorder/disease of any kind may affect the HRV parameters.
In addition, subjects associated with a medical background (preparing for/students of medical and paramedical courses and immediate family having a medical, paramedical background/profession etc.) were excluded as it might have influenced their responses in the KAP questionnaire.
METHODOLOGY
The procedures are conducted by following all the COVID-19 safety protocols. The room or the laboratory where the study was conducted and was adequately sanitized with sodium hypochlorite before and after the tests were done. The subjects were accompanied by their parents or guardians during the study.
KAP questionnaires were given to all the participants – a coinvestigator was present on hand to explain the components, clarify any doubts, and receive the responses. The responses were collected and the CVF tests were done.
The following FITs (fitness indicator tests) were done:
Body mass index (BMI)
Each subject’s height and weight were measured using stadiometer. BMI is calculated using the standard formula BMI = weight (kg)/height (sq.m). Based on the WHO criteria,[25] subjects should be classified as underweight with a BMI < 18.5 kg/m2, normal as having BMI between 18.5 and 24.9 kg/m2; overweight as BMI 25.0–29.9 kg/m2, and obese as BMI ≥30.0 kg/m2.
ABI
Using the department’s existing equipment, we customized the ABI apparatus. Before the process began, each participant was requested to lay in a supine posture. Two sphygmomanometers were linked in series and connected to a common inflation slot. One cuff was connected to the arm and the other to the ankle, and the blood pressure was recorded simultaneously. Following the measurement of the blood pressure, the individual was instructed to conduct a moderate activity and the recordings were retaken.
As per the recommendations of the IEC, ESIC Medical College, Hyderabad, VO2 Max was also added as a parameter to test CVF.
Harvard step test (HST)
A wooden block of height 16 inches was used for the exercise. Each participant was given few minutes to relax before being instructed in their native language to continuously step up and down a wooden block at a rate of 30 completed steps per minute (1 s up and 1 s down) for 5 min or until exhaustion. Exhaustion was regarded as a student’s inability to maintain a 15-s stepping pace. The time of exhaustion was recorded.
Following the conclusion of the test, the subject was made to rest for a minute and the heartbeats were counted for 30 s intervals 3 times, that is, from 1 to 1.5, 2 to 2.5, and 3 to 3.5 min.
The heart beats were counted using stopwatch and the total number of heart beats were calculated using the formula te × 100/hb × 2, where te represents the time of exhaustion; hb represents total number of heart beats.[26]
HRV
The room was maintained at normal temperature and illumination with a quiet environment. Subjects were asked to lie down on the couch for 5 min to acclimatize to the new environment, otherwise which might affect the ECG rhythm. We began our process once they were at ease.
The ECG from Lead II has been selected for recording. Lead II is preferred because it is parallel to the cardiac axis. The electrodes in Lead II are attached to the right arm and left foot, with the left leg electrode connected to the positive terminal and the right arm electrode connected to the negative electrode of the ECG machine. The MLA1090 electrode cream was applied to the right arm, left leg, and the right leg to which the ground electrode was connected. We used MLA2505 shielded lead wires with MLA700 Reusable ECG electrodes and MLA2540 5 Lead shielded bio amp cable with power lab 15T to connect to the subject.
Now, we requested the individuals to close their eyes and relax for 5 min without moving. Power lab 15T provided with the Lab Chart Pro version 8.1.19. was used to acquire the ECG data and for further analysis. The HRV software module in the system is a robust one which has the option for automatic deletion of ectopic beats. The collected data of all the subjects were saved as a file in the separate folder. The data obtained are assessed for the following ECG characteristics such as time domain and frequency domain.
Time domain parameters
Standard deviation of RR interval (SDNN): The standard deviation of all R-R intervals normalized is SDNN. The R-R intervals and beats are measured by SDNN, which has an impact on heart functioning.
Root mean square of standard deviation (RMSSD): The root mean square of the variations in normal heartbeats across time represents RMSSD. RMSSD is a measurement of the heart’s vagus-mediated regulation. RMSSD is frequently used by professional athletes to track heart activity and HRV modification in response to physical exertion. Depending on age and health, the average RMSSD ranges from 20 to 80 ms.
pNN50: The percentage of adjacent NN intervals that differ from each other by more than 50 ms. The pNN50 is closely correlated with PNS activity.
Frequency domain parameters
The distribution of absolute or relative power is estimated in four frequency bands using frequency domain measurements. The signal energy found inside a frequency band is referred to as power. It comprises both relative and absolute power. Absolute power is computed by multiplying ms squared by the number of cycles per second. The absolute power for a single frequency band is divided by the summed absolute power of the low frequency (LF) and high frequency (HF) bands to calculate relative power, which is expressed as a percentage of total HRV power or in normal units (nu). Frequency domain includes parameters such as VLF, HF, LF, and LF/HF ratio.
VO2 max
The highest amount of oxygen an individual can take in, transport, and utilize to produce ATP aerobically during heavy exercise. Rockport’s Fitness Walking test was done. The formula used for calculating was:
VO max = 132.853 – (0.0769 × body weight) – (0.3877 × age in years) + (6.3150 × sex) – (3.2649 × walk time in minutes) – (0.1565 × heart rate in beats/min). Males were coded as “1” and females as “0” for substitution of gender in the formula.[27]
KAP QUESTIONNAIRE
Personal information and other demographic data such as name, age, address, and occupation were collected separately. Each subject was given a code which was mentioned at the top of the KAP questionnaire along with the group they belong to: “U” for Urban and “R” for Rural.
| Group: U/R | ||||||
|---|---|---|---|---|---|---|
| S. No. | Questions | OPTIONS | ||||
| 1. | Regular physical activity can improve health and reduce disease risk | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 2. | Regular intake of Fresh fruits can improve health and reduce the disease risk | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 3. | Adding excess salt to food and fruits is harmful for health | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 4. | Frequent intake of outside food, especially deep fried items is bad for health | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 5. | Regular intake of fresh vegetables can improve health and reduce the disease risk | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 6. | Alcohol intake is not good for heart health | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 7. | Smoking is not good for heart health | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 8. | Sitting for longer times is good for health | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
| 9. | In your opinion, can people control their alcohol intake and not get addicted so easily? | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
| 10. | Teenage smoking is fine and people leave this habit when they grow up? | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
| 11. | It is alright to add more salt to fruits and other foods to make it tastier | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
| 12. | People make unnecessary issue of heart health and disease and that the actual issue is very small? | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
| 13. | All correct and verified information should be passed onto friends and family so that they can take better care of themselves? | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 14. | How many days per week do you eat fruit? | Daily | 5–6 | 3–4 | 1–2 | <1 |
| 15. | How many days per week do you a pure vegetarian diet? | Daily | 5–6 | 3–4 | 1–2 | <1 |
| 16. | How many times per week do you eat outside meals (meals not prepared at home/hostel)? | <1/month | <1/week | 2–3 | 4–5 | 6–7 |
| 17. | Do you add excess salt to your food after its cooked? | No | Yes | |||
| 18. | Do you smoke? (includes cigarettes, cigars, handrolled cigarettes, etc.) | No. If no, go to Q20 | Yes | |||
| 19. | If yes, specify the quantity smoked per day | 1–2 | 2–5 | 5–10 | 10–20 | >20 |
| 20. | Did you smoke in the past year? | No | Yes | |||
| 21. | Do you chew tobacco? | No. If no, go to Q23 | Yes | |||
| 22. | If yes, please specify amount used | |||||
| 23. | Do you drink alcohol? (local liquor like sendi, beer, wine, whiskey etc.) | No. If no, go to Q25 | Yes | |||
| 24. | If yes, specify the frequency of intake per week | <1/month or on special occasions | <1/week | 1–3 | 4–6 | Daily |
| 25. | Did you drink in the past 30 days? | No | Yes | |||
| 26. | How many of your waking hours per day are spent in sitting or lying down e.g., in talking to family and friends, watching TV, Phone, Tablets, Laptop, travelling etc. (Please exclude your sleep time) | <5 | 5–6 | 7–8 | 9–10 | >10 h |
| 27. | Do you perform moderate intensity physical activity for at least 10 min continuously per week which cause slight shortness of breath and awareness of heart beat? (e.g., brisk walk/bicycle) | Yes | No. If no, go to Q29 | |||
| 28. | If yes, specify the frequency (activity done on number of days/week) | Daily | 5–6 | 4–5 | 2–3 | <1 |
| 29. | Do you take part in sports regularly? | Yes | No | |||
| 30. | If yes, please specify the frequency (activity done on number of days/week) | Daily | 5–6 h/day | 4–5 h/day | 2–3 h/day | <1 h/day |
| 31. | How long does each sports session last? | >45 min | 30–45 min | 15–30 min | 5–15 min | <5 min |
OBSERVATIONS AND RESULTS
After completion of the tests, the data were collected and entered into an MS-Excel worksheet. Data were analyzed using GraphPad Prism Software (Version 9.2.0 [322]). Student’s “t” test (unpaired, two-tailed) was done and “P” < 0.05 was considered to be significant. The following results were obtained.
Means and Standard Deviation of ABI before exercise was 1.078 ± 0.064 and 1.010 ± 0.1366 for urban and rural population, respectively, with “P” = 0.0251. This indicates that there is a significant difference between the values of ABI of both groups [Tables 1-5 and Graphs 1-5].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 15.45±0.39 | 0.2376 |
| Rural | 14.38±2.87 |
SD: Standard deviation
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 20.07±4.03 | 0.2422 |
| Rural | 18.57±2.709 |
SD: Standard deviation
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 1.078±0.064 | 0.0251 |
| Rural | 1.010±0.1366 |
SD: Standard deviation
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 0.9922±0.107 | 0.0178 |
| Rural | 1.091±0.097 |
SD: Standard deviation
| Population | Mean±SD | P-value | |
|---|---|---|---|
| Preexercise | Postexercise | ||
| Urban | 1.078±0.064 | 0.9922±0.107 | <0.0001 |
| Rural | 1.010±0.1366 | 1.091±0.097 | 0.1196 |
SD: Standard deviation
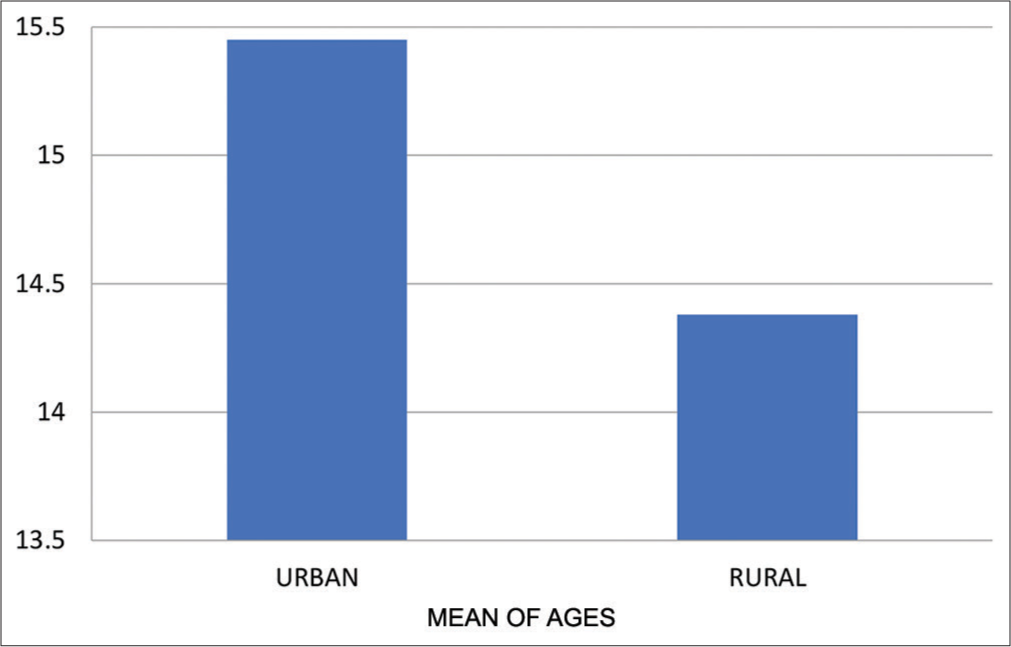
- Means of age for urban and rural group.

- Means of BMI for urban and rural group. BMI: Body mass index.

- Means of ABI at rest for urban and rural group. ABI: Ankle Brachial Index.
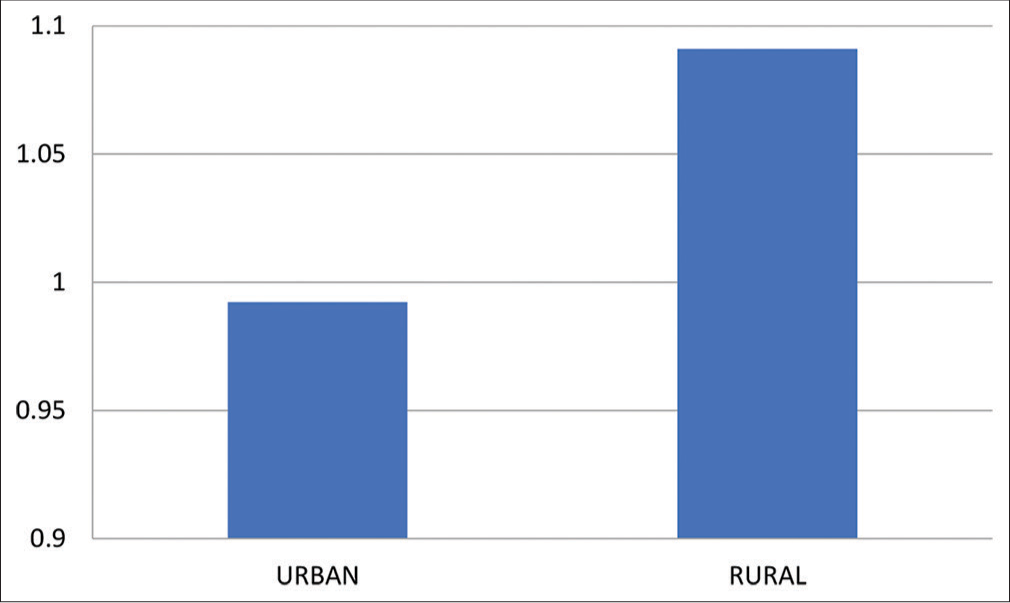
- Means of ABI post-exercise for urban and rural group. ABI: Ankle Brachial Index.

- Means of Ankle Brachial Index fo-r urban and rural group pre- and post-exercise.
Means and Standard Deviation of ABI after exercise was 0.9922 ± 0.107 and 1.091 ± 0.097 for urban and rural population, respectively, with “P” = 0.0178. This indicates that there is a significant difference between the values of ABI of both groups [Table 4 and Graph 4].
Means and Standard Deviation of ABI in both groups before and after exercise is shown in the Table 6. There is a significant fall in ABI in the urban girls group with “P” < 0.0001. In contrast, though rural group shows a rise, it is not significant, with “P” = 0.1196 [Table 6 and Graph 6].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 39.26±3.21 | 0.0066 |
| Rural | 59.55±1.403 |
SD: Standard deviation

- Means of Harvard Step Test for urban and rural group.
Means and Standard Deviation of SDNN was 37.24 ±16.88 ms and 51.64 ± 29.32 ms in urban and rural population, respectively, with “P” = 0.051, which was not significant [Table 7 and Graph 7].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban (ms) | 37.24±16.88 | 0.051 |
| Rural (ms) | 51.64±29.32 |
SD: Standard deviation

- Means of HRV – SDNN for urban and rural group. HRV: Heart rate variability, SDNN: Standard deviation of RR interval.
Similarly, Means and Standard Deviation for RMSSD for urban population was 50.22 ± 28.007 ms and it was 71.88 ± 48.49 ms for rural population, having “P” = 0.08, which was not significant [Table 8 and Graph 8].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban (ms) | 50.22±28.007 | 0.08 |
| Rural (ms) | 71.88±48.49 |
SD: Standard deviation

- Means of HRV – RMSSD for urban and rural group. HRV: Heart rate variability, RMSSD: Root mean square of standard deviation.
Means and Standard Deviation for pNN50 for urban population was 27.93 ± 2.35 and it was 43.71± 3.312 for rural population, having “P” = 0.1027, which was not significant [Table 9 and Graph 9].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 27.93±2.35 | 0.1027 |
| Rural | 43.71±3.312 |
SD: Standard deviation
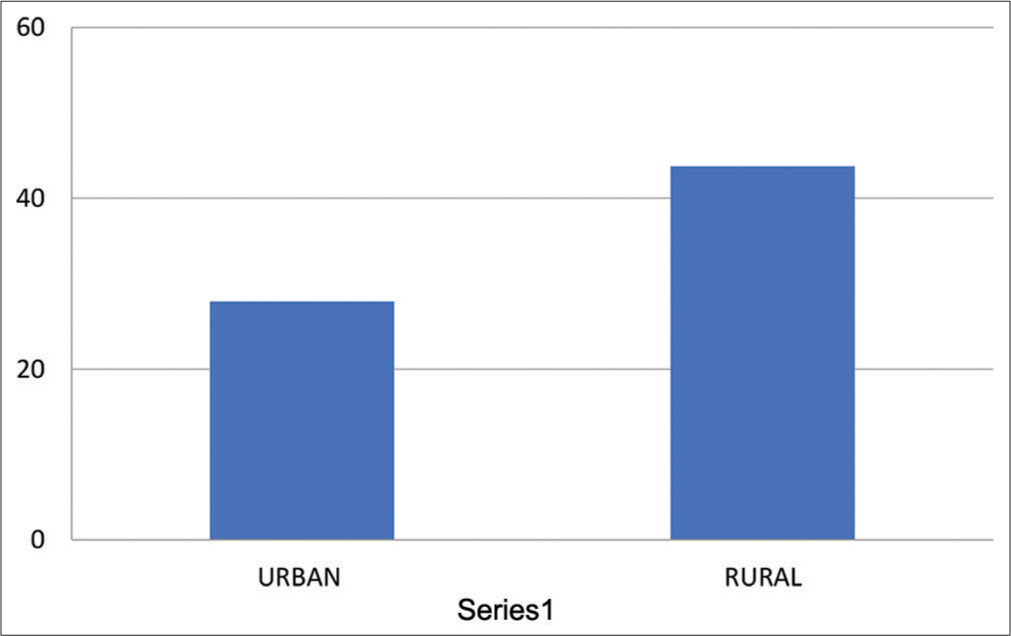
- Means of HRV – pNN50 for urban and rural group. HRV: Heart rate variability, pNN50: The percentage of adjacent NN intervals that differ from each other by more than 50 ms.
Means and Standard Deviation of low power expressed as a percentage (in LF%) was 23.83 ± 9.16 and 14.71 ± 4.4% in urban and rural population, respectively, which had “P” = 0.0082, which was significant [Table 10 and Graph 10].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 23.83±9.16 | 0.0082 |
| Rural | 14.71±4.4 |
SD: Standard deviation

- Means of HRV – LF% for urban and rural group. HRV: Heart rate variability, LF: Low frequency.
Means and Standard Deviation of high power expressed as a percentage (in HF%) was 59.24 ± 11.09 and 82.2 ± 21.03% in urban and rural population, respectively, which had “P” = 0.001, which was significant [Table 11 and Graph 11].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 59.24±11.09 | 0.001 |
| Rural | 82.2±21.03 |
SD: Standard deviation

- Means of HRV – HF% for urban and rural group. HRV: Heart rate variability, HF: High frequency.
Means and Standard Deviation of LF/HF ratio was 0.46 ± 0.343 and 0.2248 ± 0.0082 in urban and rural population, respectively, which had a “P” = 0.048, which was significant [Table 12 and Graph 12].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 0.46±0.343 | 0.048 |
| Rural | 0.2248±0.0082 |
SD: Standard deviation

- Means of LF/HF ratio for urban and rural group. LF: Low frequency, HF: High frequency.
Similarly, power expressed in nu in both LF and HF bands was also analyzed. LF power band showed mean values and Standard Deviation of 27.21 ± 11.04 and 16.55 ± 5.01 for urban and rural population, respectively, having “P” = 0.0124, which is significant. [Table 13 and Graph 13]
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 27.21±11.04 | 0.0124 |
| Rural | 16.55±5.01 |
SD: Standard deviation

- Means of LF (nu) for urban and rural group. LF: Low frequency.
HF power band showed mean values and Standard Deviation of 66.31 ± 10.6 and 75.34 ± 7.15 for urban andrural population, respectively, having “P” = 0.0243, which is significant [Table 14 and Graph 14].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 66.31±10.6 | 0.0243 |
| Rural | 75.34±7.15 |
SD: Standard deviation

- Means of HF (nu) for urban and rural group. HF: High frequency.
Regarding VO2 max, the values of Mean and Standard Deviation are 70.06 ± 1.017 and 75.42 ± 2.942 for urban and rural population, respectively, having “P” = 0.0003, which is significant [Table 15 and Graph 15].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 70.06±1.017 | 0.0003 |
| Rural | 75.42±2.942 |
SD: Standard deviation

- Means of VO2 max for urban and rural group.
Regarding KAP questionnaire scores, the values of Mean and Standard Deviation are 3.838 ± 0.28 and 3.92 ± 0.258 for urban and rural population, respectively, having “P” = 0.8518, which is significant [Table 16 and Graph 16].
| Population | Mean±SD | P-value |
|---|---|---|
| Urban | 3.838±0.28 | 0.8518 |
| Rural | 3.92±0.258 |
SD: Standard deviation

- Means of values from KAP questionnaire for urban and rural group. KAP: Knowledge, attitude, and practices.
| Classification of BMI.[25] | |
|---|---|
| BMI values | Category |
| <18.5 | Underweight |
| 18.5–24.9 | Normal |
| 25–29.9 | Overweight |
| >30 | Obese |
BMI: Body mass index
| Classification of Harvard Step Test.[26] | |
|---|---|
| HST values | Category |
| >96 | Excellent |
| 83–96 | Good |
| 68–82 | Average |
| 54–67 | Low average |
| <54 | Poor |
HST: Harvard Step Test
DISCUSSION
The current study was conceptualized to see the difference between physiological parameters of rural and urban adolescent girls, which are indicative of cardiovascular health. With lifestyle contributing significantly to health as well as development of diseases, this study is an appropriate one to note the changes which occur due to two very different lifestyles – the urban and the rural. Primordial prevention refers to prevention of development of risk factors. If this is done correctly then the risk of development of diseases and their subsequent complications can be prevented.
The aim of the study was to see the differences between the following parameters between the urban and rural adolescent girls – BMI, ABI, HST, and HRV. They are considered to be indicators of CVF.[3,4,28] Usually, KAP, which depend on various social, cultural, and economic factors also play a significant role in cardiovascular health, as has been observed in many studies in various fields.[29] Hence, they were also assessed in this study. The study began after getting approval from the Institutional Ethics Committee (IEC), ESIC Medical College, Sanathnagar, Hyderabad. The 100 apparently healthy adolescent girls between the ages 12 and 19 were chosen as subjects, although the actual age range of the subjects who agreed to participate in this study was between 13 and 17 years only. All the subjects were school going girls, although mode of teaching was varied and disrupted due to the ongoing COVID-19 pandemic. Subjects with known CVDs, obesity, and any other chronic diseases were excluded from the study. Subjects associated with a medical background (preparing for/students of medical and paramedical courses, immediate family having a medical, paramedical background/profession, etc.) were excluded as it might have influenced their responses in the KAP questionnaire. Of the 100 subjects, 50 belonged to an urban area (residents of Hyderabad city for at least 1 year) and 50 belonged to rural areas (residents of rural areas near Hyderabad for at least 1 year). Procedure and significance of the study were explained to all of them in their native language, following which they were invited to be part of the study. Informed consent was obtained from all the participants and their parents/guardians. A questionnaire was designed to know the level of KAP of the subjects. Internal validation of the questionnaire was done with a Cronbach’s alpha score of 0.7.
Questions were categorized as those which tested their knowledge about health and risk factors for development of disease, their attitude toward them and the day-to-day practices observed by the subjects. In the questionnaire, eight questions were related to knowledge, five on attitude, and 12 questions on practices followed by them. Additional questions were present to get more information about a particular practice, if it was done or practiced by the respondent. Scoring was done for the questions ranging from 5 to 1. For the sake of simplicity and ease of calculation, the questions were framed in such a way that the better response was always 5, be it placed in any order in the multiple-choice options. This was helpful not only in our calculations, but also removed the predictability factor for respondents. The responses were collected and analyzed. Mean or average score of each subject was calculated and from this mean of each group was calculated with possible values of the average group score ranging between 1 and 5. CVF tests and measurements such as BMI, ABI, HST, HRV, and VO2 max were performed and their results were analyzed.
The results of the above-mentioned tests as well as the KAP questionnaire were obtained and entered in MS-Excel Worksheet. Statistical analysis was done using GraphPad Prism Software (Version 9.2.0 [322]). Student’s “t” test (unpaired, two-tailed) was done and “P” < 0.05 was considered to be significant.
The results indicated that the BMI was within normal range in both groups and was slightly higher in urban adolescent girls compared to rural girls, although the difference was not significant, with “P” = 0.22. Such a difference between the urban and rural groups was also seen in a study done by Goyal, which recorded higher mean BMI values in the urban group.[30]
ABI was recorded at rest and after making the subjects walk on the treadmill at a speed of 2.4 kmph for 5 min or till subjects got exhausted.[31] Subjects were familiarized with the functioning of the treadmill beforehand. It is recommended that any changes in the post-exercise ABI are better indicators of presence of peripheral arterial disease, which include a fall of ABI to <0.9 or a 20% fall in ABI values or a fall of ankle pressure by 30 mm post-exercise.[31] In our study, ABI before exercise was higher for urban population compared to the rural population with “P” = 0.0251, which is significant. This result is in line with that observed in a study done on urban and rural population in Central African countries, where the rural group had better ABI.[32]
Conversely after exercise, the ABI was higher for the rural population, respectively, with “P” = 0.0178, which is significant. This indicates that there is a significant difference between the values of ABI of both groups, although post-exercise changes seem to be a better indicator.
Comparison was also done before and after exercise in both groups. The urban girls group had a fall of ABI post-exercise by 8.6% with “P” < 0.0001, which is extremely significant. It is interesting to note here that even though the difference in means shows significance, the actual fall which can be taken as the cutoff to indicate the presence of peripheral artery disease (PAD) is <20%.[31] Although a decrease in ABI is seen in urban girls post exercise, it is not indicating the presence of an actual PAD.
The rural girls group had a rise of ABI post exercise with “P” = 0.11, which is not statistically significant. Yet, some studies point out that a rise in ABI may also be an indicator of risk for the future cardiovascular events.[33] However, most studies report that an increased ABI is an indicator of CVF.[34]
ABI is an excellent indicator of presence of peripheral arterial disease be it recorded at rest or post-exercise. ABI values ≤0.9 are very good indicators for presence of lower extremity arterial stenosis.[35] Kajikawa et al. have shown an increase in endothelial dysfunction in association with low and borderline ABI.[36] Various studies done by multiple researchers have shown an association between low values of ABI with a higher risk of stroke, myocardial infarction, other cardiovascular morbidities, and mortalities.[37-39] In light of such observations, it is a very valuable indicator for CVF for our study.
Rural girls performed better in HST than the urban girls with “P” = 0.0066, which is very significant. It is to be noted that even though rural group performed better, their mean values still fall under the category of low-average as per HST rating whereas the means of urban group falls under poor category. In a study done by Mahmood et al. comparing urban and rural physiotherapy students, it was seen that the urban group performed better than the rural group.[40] Our study does not support this. Conversely, in studies done by Gahlawat[41] and by Biraadar,[42] it was seen that cardiovascular efficiency as measured by HST was greater in rural high school girls compared to urban high school girls with rural population having a better score of HST compared to the urban group. This is similar to our study.
HRV has also been measured in all the subjects. The parameters of HRV analyzed include time domains such as SDNN, RMSSD, frequency domains such as LF, HF, and LF/ HF Ratio.
Regarding HRV, the time domain parameters such as SDNN, RMSSD, and pNN50 were higher in rural girls, but this difference was not significant.
RMSSD indicates the beat-to-beat variance of the heart rate. It is considered to be the primary time-domain measure for estimating the vagally mediated changes as observed in HRV.[43,44] It is correlated with HF power.[43,45] Closely associated with HF power is the pNN50. In addition, it was observed that a correlation is also seen between pNN50 and RMSSD.[43,46]
Therefore, RMSSD, SDNN, and pNN50 are measures of autonomic vagal influence on the heart, with lower values indicating shift in autonomic activity away from the vagus[47] and toward greater sympathetic activity.[43]
Our study shows greater time domain values for the rural group compared to the urban group, although the rise in not significantly higher. Greater time domain values have been associated with aerobic fitness and vice-versa.[48]
LF was significantly higher in the urban girls group, compared to the rural group whereas HF and LF/HF ratio was significantly higher in the rural group. The environment can probably be playing a role as evidenced by a study done by Joung et al., in which even a very short-term exposure to the countryside lead to increased HF and lowered LF values.[49] Urban environment has higher degrees of noise and air pollution both of which are linked to decreased HF and increased LF, as well as decreased time domain parameters such as RMSSD and SDNN as evidenced by a study done by Rita Biel et al. These changes which were seen after a short-term exposure are likely to persist if the exposure continues.[50]
Although it is seen that LF shows influences of both SNS and PNS, it is traditionally considered to reflect activity of SNS primarily. On the other hand, the HF band is related to parasympathetic activity and HF power indicates degree of vagal modulation of the heart and heart rate.[43]
Decrease in HF power indicates lesser vagal tone and has been associated with stress, anxiety, etc. Modulating the vagal tone helps in maintaining the CVS health with decreased tone leading to increased morbidity.[43,51]
As mentioned above, traditionally, LF is associated with SNS and HF associated with PNS. Hence, when we consider the LF/HF ratio, a lower ratio indicates parasympathetic dominance and vice-versa.[43]
ANS dysfunction is associated with many disorders, including CVD and its associated morbidities and mortalities.[48] HRV is an important tool to assess autonomic cardiac dysregulation, which plays a role in development of pathological processes leading to a decline in health.[43]
VO2 max was a parameter added as per the recommendations of the IEC, ESIC Medical College, Sanathnagar, Hyderabad to assess CVF. It is considered to be an indicator of cardiorespiratory fitness.[27] Significantly higher values for VO2 max were obtained for the rural group compared to the urban group. In a study done by Suleiman et al., it was observed that there is no significant difference between the VO2 max values of urban and rural study subjects.[52]
However, the results of our study were similar to an Indian study done which compared the difference in VO2 max values in urban and rural girls and showed a significant increase in the values of VO2 max of the rural girls.[53]
KAP questionnaire questions were remodeled for the sake of clarity. Internal validation was done and a Cronbach’s alpha score of 0.7 was obtained. Questions were asked to know their level of knowledge, their attitude toward health and disease and the daily practices done which may contribute toward CVS health. The results were analyzed. Mean scoring of all the questions answered by a subject was done and means of the both the groups were assessed from this. The possible values were expected to range between 1 and 5. Surprisingly, the mean values of both groups were very similar with little difference between them and an insignificant “P” value. We believe that with the advent of better schooling opportunities and accessibility of smartphones and internet, both urban and rural girls had similar level of knowledge and attitudes along with a similar impact on practices followed by them in their daily life.
Our study shows slightly better results of the physiological parameters in the rural population.
CONCLUSION
The distinction and final labeling of whether urban or rural lifestyle is not an easy one, with our own study not clearly showing absolute results favoring one over the other. Parameters such as BMI were non-indicative with no significant difference between the values of both groups. ABI showed varied results, with urban girls scoring more than rural girls at rest and rural girls scoring more post-exercise compared to the urban population. When the mean of each group is compared at rest and post-exercise, urban girls showed a significant decrease of score after exercise compared to their pre-exercise values; though this drop is not enough to be indicative of peripheral vascular disease. On the other hand, the ABI values of rural girls increased after exercise compared to that at rest, yet this rise was not significant. The values of HST are better in the rural population. Similarly, even HRV shows better results in the rural population with the frequency domain parameters being significantly better in the rural girls. Although time domain values are also greater in rural girls compared to the urban girls, this rise is not significant. KAP questionnaire values showed surprising similarities in both the groups. This can be attributed to better opportunities to schooling, greater exposure to internet which may have contributed to better awareness about health. It appears that with similar KAP, it is the environmental influences such as air and noise and nutritional influences as seen by BMI, which may account for some of the changes in the above tested physiological parameters. Adolescence is the period of change, growth, and development. This is also the period of vulnerability with risk factors marking their presence and increasing the likelihood of a person developing a disease in later life. Hence, primordial prevention when applied at this younger age can be beneficial, as it prevents the development of risk factors.
Summary
This study was conceptualized to determine any difference in the physiological parameters determining cardiovascular health in adolescent girls of the urban and rural areas. Clearance for this study was given by the Institutional Ethics Committee, ESIC Medical College, Sanathnagar, Hyderabad. The aim of the study was to see the differences between the following parameters between the urban and rural adolescent girls – BMI, ABI, HST, and HRV. They are considered to be indicators of CVF.[3,4,28] A questionnaire was designed to know the level of KAP of the subjects. Internal validation of the questionnaire was done with a Cronbach’s alpha score of 0.7.
The 100 apparently healthy adolescent girls between the ages 13–17 agreed to participate in this study, of which 50 belonged to an urban area and 50 belonged to rural areas. Subjects with known CVDs, obesity, and any other chronic diseases or a medical background were excluded from the study. Procedure was explained and informed consent was obtained from all the participants and their parents/ guardians. The results were variable, with BMI having non-significant higher values in urban girls, ABI showing better post-exercise values in rural girls, HST and HRV showing better responses in rural girls. KAP questionnaire showed very similar responses, which can be attributed to improved schooling opportunities and better access to internet and smartphones which can increase awareness. Hence, the likely reason for better performance of rural girls could be the environment around them with cleaner and fresher air, lesser noise, and lesser weight compared to the urban girls, although the weight is in normal range for both. All these factors might have contributed to better CVF in the rural population.
Limitations of the study
KAP questionnaire is subjective to the actual veracity of the responses given by the subjects.
The reasons behind the differences in the physiological parameters in urban and rural group need to be tested at a molecular level, which is beyond the scope of this study. Based on similar studies, it can be deducted that environmental influences play a huge role in determining the CVF.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Comparison of cardiovascular endurance and speed between urban and rural female students of Bahauddin Zakariya University Pakistan. Int J Res. 2017;5:361-6.
- [CrossRef] [Google Scholar]
- Health related physical fitness of boys age 8-18 years. J Exerc Sci Physiother. 2005;1:12-22.
- [Google Scholar]
- High cardiovascular fitness is associated with low metabolic risk score in children: The European youth heart study. Pediatr Res. 2007;61:350-5.
- [CrossRef] [PubMed] [Google Scholar]
- Relations of total physical activity and intensity to fitness and fatness in children; The European youth heart study. Am J Clin Nutr. 2006;84:299-303.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial compliance in young children: The role of aerobic fitness. Eur J Cardiovasc Prev Rehabil. 2005;12:492-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular health decline in adolescent girls. Prev Med Rep. 2020;20:101276.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164:1092-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rural-urban comparison in prevalence of overweight and obesity among children and adolescents of Asian Indian origin. Asia Pac J Public Health. 2011;23:928-35.
- [CrossRef] [PubMed] [Google Scholar]
- Adolescent girls' health, nutrition and wellbeing in rural eastern India: A descriptive, cross-sectional community-based study. BMC Public Health. 2019;19:673.
- [CrossRef] [PubMed] [Google Scholar]
- Rural-urban differences in body mass index and obesity-related behaviors among low-income preschoolers. J Public Health (Oxf). 2020;43:e637-44.
- [CrossRef] [PubMed] [Google Scholar]
- Urban rural comparison of anthropometry and menarcheal status of adolescent school going girls of Jodhpur, Rajasthan, India. J Clin Diagn Res. 2016;10:SC08-12.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study on nutritional status of urban and rural early adolescent school girls of West Bengal, India. J Nepal Paediatr Soc. 2011;31:169-74.
- [CrossRef] [Google Scholar]
- Utility of ankle-brachial index in screening for peripheral arterial disease in rural India: A cross-sectional study and review of literature. Indian Heart J. 2018;70:323-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of peripheral artery disease and associated risk factors in a Brazilian rural population: The Baependi heart study. Int J Cardiovasc Sci. 2018;31:405-13.
- [CrossRef] [Google Scholar]
- Post-exercise ankle-brachial index is not an indispensable tool for the detection of peripheral arterial disease in an epidemiological survey. A post-hoc analysis of the Athens study. Int Angiol. 2013;32:518-25.
- [Google Scholar]
- Trends in urban/rural inequalities in cardiovascular risk bio-markers among Chinese adolescents in two decades of urbanisation: 1991-2011. Int J Equity Health. 2018;17:101.
- [CrossRef] [PubMed] [Google Scholar]
- Urban-rural differences in cardiovascular disease risk factors: A cross-sectional study of schoolchildren in Wuhan, China. PLoS One. 2015;10:e0137615.
- [CrossRef] [PubMed] [Google Scholar]
- Performance of different adiposity measures for predicting cardiovascular risk in adolescents. Sci Rep. 2017;7:43686.
- [CrossRef] [PubMed] [Google Scholar]
- Heart rate variability in adolescents: Relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc. 2005;37:1856-63.
- [CrossRef] [PubMed] [Google Scholar]
- Heart rate variability and cardiovascular risk factors in adolescent boys. J Pediatr. 2014;165:945-50.
- [CrossRef] [PubMed] [Google Scholar]
- Urban rural differences in diet, physical activity and obesity in India: Are we witnessing the great Indian equalisation? Results from a cross-sectional STEPS survey. BMC Public Health. 2016;16:816.
- [CrossRef] [PubMed] [Google Scholar]
- Rural adolescent health: Issues, behaviors and self-reported awareness. J Community Med Health Solut. 2020;1:1-17.
- [CrossRef] [Google Scholar]
- Comparison of world health organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2465-75.
- [CrossRef] [PubMed] [Google Scholar]
- Stepping from Belgium to the United States and back: The conceptualization and impact of the Harvard step test, 1942-2012. Res Q Exerc Sport. 2013;84:186-97.
- [CrossRef] [PubMed] [Google Scholar]
- Smith, Exercise Physiology for Health, Fitness, and Performance (4th ed). Philadelphia, PA: Lippincott Williams and Wilkins, a Wolters Kluwer Business; 2014.
- [Google Scholar]
- Cardiorespiratory endurance evaluation using heart rate analysis during ski simulator exercise and the Harvard step test in elementary school students. J Phys Ther Sci. 2016;28:641-5.
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge, attitude, and practice regarding cardiovascular diseases in adults attending health care centers in Tehran, Iran. Int J Endocrinol Metab. 2020;18:e101612.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of nutritional status of rural and urban adolescent girls from schools in North India: A cross-sectional study. Int J Community Med Public Health. 2018;5:1996-2002.
- [CrossRef] [Google Scholar]
- Comparison of different exercise ankle pressure indices in the diagnosis of peripheral artery disease. Vasc Med. 2018;23:541-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of peripheral artery disease in the elderly population in urban and rural areas of Central Africa: The EPIDEMCA study. Eur J Prev Cardiol. 2015;22:1462-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of an increase in post-exercise ankle-brachial index. Vasc Med. 2017;22:204-9.
- [CrossRef] [PubMed] [Google Scholar]
- The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med. 2006;166:529-35.
- [CrossRef] [PubMed] [Google Scholar]
- Change in ankle-brachial index over time in a screened Japanese cohort-the Okinawa peripheral arterial disease study. Circ J. 2016;80:2255.
- [CrossRef] [Google Scholar]
- Borderline ankle-brachial index value of 0.91-0.99 is associated with endothelial dysfunction. Circ J. 2014;78:1740-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197-208.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of peripheral artery disease on prognosis in hospitalized heart failure patients. Circ J. 2015;79:785-93.
- [CrossRef] [PubMed] [Google Scholar]
- Poor prognosis in critical limb ischemia without preonset intermittent claudication. Circ J. 2015;79:1618-23.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of physical fitness between rural and urban physical therapy students studying in Lahore, Pakistan. APMC. 2018;12:85-9.
- [Google Scholar]
- Comparison of physical fitness status of rural and urban male collegiate students in Kurukshetra. J Exerc Sci Physiother. 2007;3:157-9.
- [Google Scholar]
- Cardiovascular efficiency and strength between rural and urban school girls. Int J Phys Educ Sports Health. 2021;8:210-1.
- [Google Scholar]
- An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
- [CrossRef] [PubMed] [Google Scholar]
- A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040.
- [CrossRef] [PubMed] [Google Scholar]
- Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88-101.
- [CrossRef] [PubMed] [Google Scholar]
- Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593-601.
- [CrossRef] [Google Scholar]
- RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP-7 inventory. Epilepsy Behav. 2010;19:78-81.
- [CrossRef] [PubMed] [Google Scholar]
- The application of heart rate variability biofeedback to medical and mental health disorders. Biofeedback. 2017;45:2-8.
- [CrossRef] [Google Scholar]
- Measures to promote rural healthcare tourism with a scientific evidence-based approach. Int J Environ Res Public Health. 2020;17:3266.
- [CrossRef] [PubMed] [Google Scholar]
- Acute cardiovascular health effects in a panel study of personal exposure to traffic-related air pollutants and noise in Toronto, Canada. Sci Rep. 2020;10:16703.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122-31.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of physical fitness of rural, semi-urban and urban of primary school children in their abdominal strength, flexibility and cardio-respiratory endurance in federal capital territory, Nigeria. MOJ Sports Med 2018:237-42.
- [Google Scholar]
- Estimation of maximum oxygen uptake by evaluating cooper 12-min run test in female students of West Bengal, India. J Hum Sport Exerc. 2013;8:1008-14.
- [CrossRef] [Google Scholar]







