Translate this page into:
Association of Vitamin D Insufficiency and Peripheral Vascular Resistance
S. Radhika, DM (Clinical Pharmacology & Therapeutics) Department of Clinical Pharmacology and Therapeutics, Nizam’s Institute of Medical Sciences Hyderabad, Telangana India dr_radhika_s@yahoo.co.in
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Vitamin D insufficiency is prevalent worldwide. Recently, the role of vitamin D substitution in hypertensive vitamin D deficient and insufficient patients has been studied by many investigators worldwide. In this observational study, the peripheral vascular resistance (PVR) of patients with vitamin D insufficiency or deficiency was analyzed.
Objective The main purpose of this article is the assessment of PVR in vitamin D deficient subjects and in that any gender variation.
Methods Subjects who were not known hypertensives, and who were free of other comorbidities, were included. Diabetic subjects were excluded. Serum vitamin D3 levels of all the subjects were recorded. Thirty-three subjects with serum vitamin D3 levels in insufficiency or deficiency range were included in our study. AGEDIO peripheral resistance calculator was used to calculate arterial stiffness indices, PVR, stroke volume, cardiac output, and cardiac index. Augmentation pressure (AG) is the contribution that wave reflection makes to systolic arterial pressure. Augmentation index (AI) is an indirect measure of arterial stiffness and is calculated as AG divided by pulse pressure ×100.
Results The total number of subjects studied was 33, of whom 18 were males, and 15 were females. The mean serum vitamin D3 levels were 19.28 nanograms/mL. In this study, it was observed that the mean AI was 21.42 ± 12.6%, and mean pulse wave velocity was 6.9 ± 1.2 m/s. Among central hemodynamic measures, mean PVR was found to be 1733 ± 201.1dyn*s/cm5, mean cardiac output was 4.3 ± 0.5 L/min, and mean cardiac index was 2.56 +/– 0.33 L/mi*L/m2). Twenty-five (76%) patients showed increased PVR. Even though in patients with normal PVR, there is female preponderance, but in patients with high PVR, there was no gender difference.
Conclusions This study has shown that the PVR was significantly high in vitamin D deficient and insufficient subjects (without hypertension or diabetes mellitus) with no significant gender difference for high PVR, even though AG and AI were not significantly different among subjects.
Keywords
vitamin D insufficiency
peripheral vascular resistance
Introduction
Vitamin D was traditionally used in therapeutics as a mediator for efficient absorption of calcium and to aid the action of parathyroid hormone. Until recently, its role in therapeutics was as a vitamin, but now it is realized that it acts more like a hormone that is synthesized in mammals. Dietary supplementation may not be required.1
Vitamin D deficiency is prevalent worldwide, both in temperate and tropical countries, irrespective of age, gender, and geography. While community-based studies in India report a prevalence of vitamin D deficiency as 50 to 94%, hospital-based studies reported a prevalence of 37 to 99%.2
Although it is well known that vitamin D deficiency affects the musculoskeletal system, evidence suggests that it may also adversely affect the cardiovascular system. Vitamin D deficiency or insufficiency is now being reported to be associated with increased arterial stiffness leading to cardiovascular morbidity.3
Recent studies showed that, even though vitamin D deficiency is related to hypertension, the mechanism is not clear and is contemplated due to its effects on peripheral vascular resistance (PVR). However, there is limited data on the association of vitamin D deficiency with PVR. This observational study was taken up to assess the effect of vitamin D insufficiency on arterial stiffness and hemodynamic parameters in patients without hypertension or diabetes.
Methodology
The study was designed following Good Clinical Practice guidelines. This is an observational study done in the outpatient clinic of cardiology.
In this study, patients of both genders with vitamin D deficiency or insufficiency were included. Patients with hypertension or diabetes or chronic illness or chronic kidney disease were excluded. The demographic data, serum vitamin D levels, blood pressure measurements, central aortic pressures, and measures of arterial stiffness were recorded.
Vitamin D deficiency is defined as a serum 25-hydroxyvitamin D level of < 20 nanograms/mL, whereas vitamin D insufficiency is regarded as a 25-hydroxyvitamin D level of between 21 and 29 nanograms/mL.
AGEDIO peripheral resistance calculator was used to calculate arterial stiffness indices, PVR, stroke volume, cardiac output, and cardiac index. Augmentation pressure (AG) is the contribution that wave reflection makes to systolic arterial pressure. Augmentation index (AI) is an indirect measure of arterial stiffness and is calculated as AG divided by pulse pressure ×100.
Statistical Analysis
The data thus obtained was presented as mean and standard deviation for quantitative variables and proportions or percentages for categorical variables. The difference in arterial stiffness between male and female patients was assessed using the unpaired Student’s t-test.
Results
In this study, 33 patients were included, with a mean age of 49.3 ± 10.72 years with a mean height of 162.27 ± 7.36 cm, which showed a significant difference between males and females. M:F was 5:6. The mean serum vitamin D3 levels were 19.28 nanograms/mL (Table 1). Serum vitamin D levels were observed to be significantly lower in females when compared with males (p = 0.04).
|
Parameter |
Male |
Female |
p-Value |
|---|---|---|---|
|
No. of subjects |
15 |
18 |
– |
|
Mean age (y) |
50.53 ± 11.12 |
48.28 ± 10.58 |
0.559 |
|
Mean height (cm) |
166.87 ± 6.55 |
158.44 ± 5.71 |
0.001 |
|
Serum vitamin D3 levels (nanograms/mL) |
22.3 ± 2.2 |
15.3 ± 0.3 |
0.04 |
Brachial (Table 2) and derived central aortic (Table 3) systolic, diastolic, and pulse pressures were comparable in genders with vitamin D deficiency subjects.
|
Parameter |
Male |
Female |
p-Value |
|---|---|---|---|
|
Abbreviation: BP, blood pressure. |
|||
|
Systolic BP (mm Hg) |
125.5 ± 3.1 |
117.8 ± 3.2 |
0.09 |
|
Diastolic BP (mm Hg) |
84.73 ± 2.36 |
80.39 ± 2.54 |
0.22 |
|
Mean arterial pressure (mm Hg) |
101.8 ± 2.8 |
96.44 ± 2.7 |
0.18 |
|
Pulse pressure (mm Hg) |
40.1 ± 1.99 |
37.2 ± 2.1 |
0.32 |
|
Parameter |
Male |
Female |
p-Value |
|---|---|---|---|
|
Abbreviations: AMP, adenosine monophosphate; BP, blood pressure; PP, pulse pressure. |
|||
|
Central aortic systolic BP (mm Hg) |
114.9 ± 3.2 |
113.8 ± 5.13 |
0.87 |
|
Diastolic BP (mm Hg) |
85.6 ± 2.4 |
81.7 ± 2.5 |
0.28 |
|
Pulse BP (mm Hg) |
29.3 ± 1.7 |
28.28 ± 1.4 |
0.65 |
Subjects in the study showed mean AG and AI of 5.55 ± 3.938 mm Hg and 21.42 ± 12.58%, respectively. AG and AI were either low or normal in 96.9% (n = 32) and 87.8% (n = 29), respectively. There was no significant difference between both genders for AG or AI (p = 0.38 and 0.18 for low and normal AG, respectively, and p = 0.40 and 0.73 for low and normal AI, respectively). High AG and AI were seen in one and four subjects, respectively. High AI was distributed equally in both genders (p = 1) (Table 4).
|
Parameter |
Male |
Female |
p-Value |
|---|---|---|---|
|
Augmentation pressure (mm Hg) |
6.67 ± 1.35 |
4.61 ± 0.5 |
0.13 |
|
Augmentation index (%) |
23.14 ± 3.21 |
21.2 ± 3.01 |
0.66 |
|
Pulse wave velocity (m/s) |
7.1 ± 0.34 |
6.8 ± 0.28 |
0.48 |
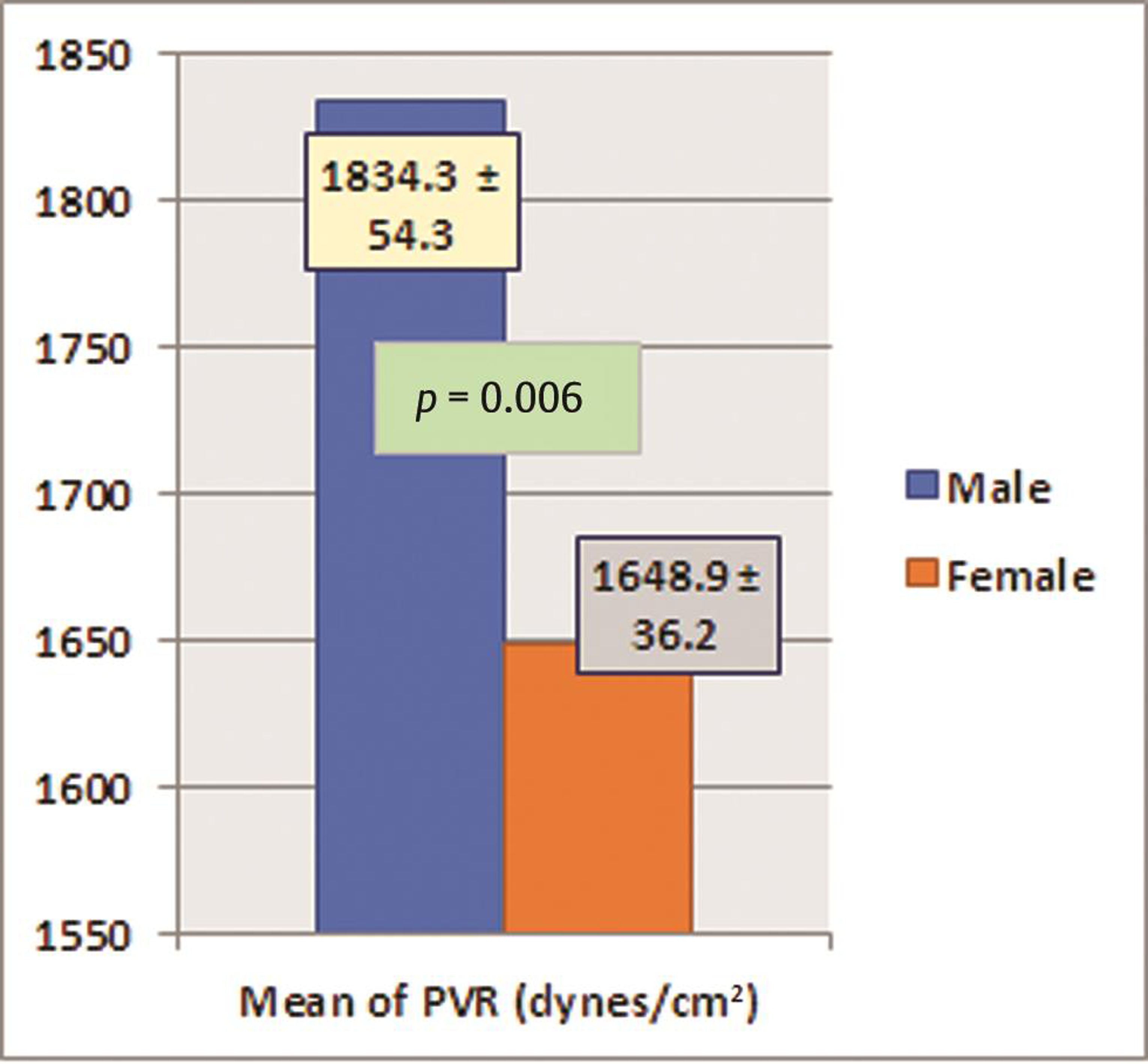
The subjects also had a mean PVR of 1,733.2 ± 201.7 dynes-cm. Among the hemodynamic parameters, only PVR was found to be significantly low in females when compared with males (p = 0.006); rest of all parameters were comparable between males and females. PVR was normal in 24% (n = 8) subjects with vitamin deficiency or insufficiency. Seventy-six percent of subjects with vitamin D deficiency or insufficiency showed increased PVR. There was no significant gender difference for increased PVR among subjects. Among all subjects with normal PVR, preponderance was significantly (p 0.021) more in females than males. Peripheral pulse wave velocity was normal or low in 45.45% (n = 15) of subjects and high in 55.55% (n = 18) of subjects. No significant difference was seen between genders for low or normal PW velocity (p = 0.1 and 0.67), whereas other parameters like heart rate, cardiac output, and stroke volume were comparable between males and females (Table 5) (Figs. 1 2).
|
Parameter |
Male |
Female |
p-Value |
|---|---|---|---|
|
Abbreviations: CI, cardiac index; CO, cardiac output; HR, heart rate; PVR, peripheral vascular resistance; SV, stroke volume. |
|||
|
HR (bpm) |
76.7 ± 2.45 |
83.3 ± 3.18 |
0.1 |
|
PVR (dynes/cm2) |
1834.3 ± 54.3 |
1648.9 ± 36.2 |
0.006 |
|
CO (L/min) |
4.2 ± 0.1 |
4.4 ± 0.1 |
0.1 |
|
SV (L) |
55.1 ± 2.22 |
54.3 ± 2.6 |
0.8 |
|
CI (L/BSA) |
2.4 ± 0.08 |
2.7 ± 0.08 |
0.09 |

-
Fig. 1 The mean augmentation pressure and index in males and females.
Fig. 1 The mean augmentation pressure and index in males and females.
-
Fig. 2 The statistical difference in peripheral vascular resistance (PVR) between males and females.
Fig. 2 The statistical difference in peripheral vascular resistance (PVR) between males and females.
Discussion
The role of vitamin D has been evolving as a mechanism of action is getting unraveled. Vitamin D in its active form, calcitriol, acts by its interaction with vitamin D receptor (VDR). It binds with cytosolic VDRs and reaches the nucleus and modifies gene transcription by interacting with deoxyribonucleic acid. Although calcium homeostasis is its primary function, the ever-increasing knowledge suggests its importance in the regulation of several other processes. It is now known that several other cells like hematopoietic cells, lymphocytes, epidermal cells, hair follicles, fat tissue, islets of pancreas, and neurons express VDRs, which indicate that it has more significant role physiologically.1
Vitamin D might influence metabolic pathways by genomic and nongenomic processes, by modulating properties of peripheral arteries. The genomic effects are implicated through angiogenesis, elastogenesis, and immunomodulation. It was observed that intravenous administration of vitamin D causes a transient increase in pulse rate and mean blood pressure, which suggests a nongenomic role of 1α,25(OH)2D3 in increasing peripheral resistance.4
Recent studies demonstrate that vitamin D has a role in the regulation of the renin-angiotensin system. In a cross-sectional study, it was observed that subjects with low vitamin D levels had high plasma angiotensin II levels when compared with subjects with normal levels of vitamin D. In another study, an inverse relation of serum 1,25(OH)2D3 level to the plasma renin activity was observed.5 Even though in the present study, the levels of angiotensin were not measured, the increase in PVR could be due to elevated angiotensin levels.
In a study, it was demonstrated that VDR null mice developed hypertension and cardiac hypertrophy. In these mice, increased gene expression of renin and plasma angiotensin II levels was observed. The effect on renin gene expression was shown to be independent of calcium metabolism in a study done on cultured cells, in which the authors of this study concluded that calcitriol negatively regulates the renin-angiotensin system. Later it was found that vitamin D binds to cyclic-adenosine monophosphate response element-binding protein, which helps in the transcription of renin messenger ribonucleic acid and inhibits expression of the renin gene.5
In addition, vitamin D deficiency is known to cause alteration of the sensitivity of vascular smooth muscle cells and can lead to hypertension.6 In a small study from Belgium of 25 patients with hypertension, vitamin D levels were inversely correlated with systolic blood pressure, diastolic blood pressure, and calf vascular resistance.7 We had taken only low vitamin D level patients without hypertension. Probably if these subjects are followed up for some time, then we can see where they are likely to develop systemic hypertension or not.
According to Santos et al’s study, supplementation of vitamin D to normotensive rats increased arterial pressure. The increased arterial pressure was found to be associated with vascular changes. Nicotinamide adenine dinucleotide phosphate oxidase, nitric oxide, and extracellular matrix components were found to have a role in modulating vascular changes.8 More studies are thus required to evaluate the effect of vitamin D supplementation on the arterial pressures and hemodynamic parameters.
As there is an increase in the incidence of vitamin D deficiency, all abnormalities seen in this state may not be due to vitamin D deficiency, including PVR. To see the real cause and effect, we selected the patients without hypertension and diabetes, which may have an effect on the PVR. Still increased PVR was very high (76%) in these vitamin D deficient patients. In our study, even though we have taken only vitamin D deficient patients, we found that females had lesser levels of vitamin D than males; males have more increased PVR.
The major limitation of the study, in addition to the sample size, is patients were not followed subsequently with or without the supplementation of vitamin D. We are not shown whether this increased PVR is reversible or not.
In conclusion, 76% of vitamin D deficient Indian subjects had increased PVR. Females had a greater degree of vitamin D deficiency, whereas male subjects had a greater increase in PVR. This study opens a new research avenue to find out whether these subjects develop hypertension early than controls and also to assess the effect of supplementation of vitamin D in this subgroup of vitamin D deficiency subjects with an increase in PVR. It is also worthwhile to pursue and correlate with the histopathologic changes associated with arterial resistance.
Conflict of Interest
None declared.
References
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics. (12th edition.). New York, NY: McGraw-Hill Education/Medical; 2011. p. :1280-1283. ISBN-10: 0071624422, ISBN-13: 978–0071624428
- [Google Scholar]
- Study of correlation of serum vitamin D levels with arterial stiffness and cardiovascular morbidity in elderly individuals of western Rajasthan. J Assoc Physicians India. 2018;66(03):18-21.
- [Google Scholar]
- Vitamin D, shedding light on the development of disease in peripheral arteries. Arterioscler Thromb Vasc Biol. 2005;25(01):39-46.
- [Google Scholar]
- The effects of vitamin D on the renin-angiotensin system. J Nephropathol. 2014;3(02):41-43.
- [Google Scholar]
- Relationship between vitamin D3 and the peripheral circulation in moderate arterial primary hypertension. Blood Press. 1994;3(06):389-393.
- [Google Scholar]
- Vitamin D induces increased systolic arterial pressure via vascular reactivity and mechanical properties. PLoS One. 2014;9(06):e98895. 10.1371/journal.pone.0098895
- [Google Scholar]







