Translate this page into:
Association between Endogenous Sex Hormones and Coronary Artery Disease in Postmenopausal Women
Dr. Roopali Khanna, MD Department of Cardiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences Raebareli Road, Lucknow -226014 India drroopalik@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
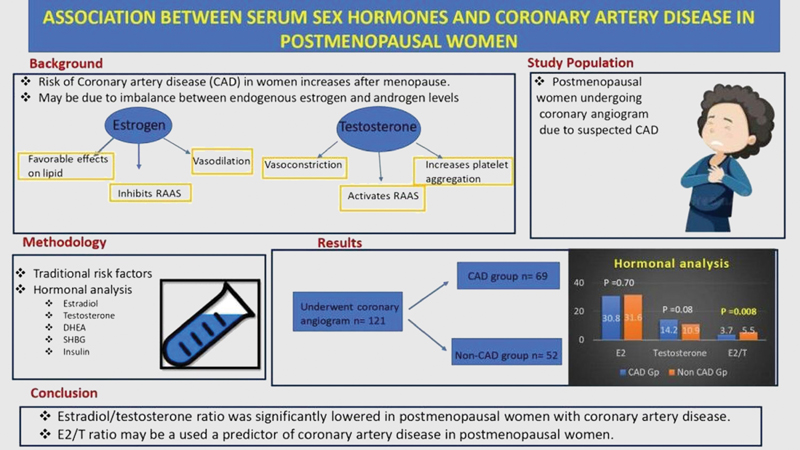
Background Incidence of coronary artery disease (CAD) increases significantly in postmenopausal women, which is assumed to be an imbalance between serum androgen and estrogen levels. However, studies assessing serum sex hormones and CAD are few and have shown conflicting results.
Objective To compare serum sex hormone levels and traditional risk factors among postmenopausal women with angiographically proven CAD and without CAD.
Method The study included consecutive postmenopausal women undergoing coronary angiography in our institute from May 2016 to June 2017. The clinical and coronary angiographic data and traditional risk factors were assessed. Fasting serum levels of estradiol (E2), testosterone (T), sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEA-S), and insulin were measured.
Results A total of 121 postmenopausal women were included in the study; 69 were CAD and 52 without CAD. Single-vessel disease was most common (55.1%), followed by double-vessel disease (24.6%) and triple-vessel disease (20.3%). Women with CAD had significantly lower estradiol/testosterone (E2/T) ratio (3.7 ± 2.6 vs. 5.4 ± 4.2, p = 0.008) compared with non-CAD group. SHBG, DHEA-S, and insulin levels were similar in CAD and non-CAD groups. The serum level of estradiol predicted the E2/T ratio (r = 0.316, p < 0.001) and positively associated with DHEA (r = 0.181, p = 0.047). Testosterone was negatively associated with E2/T ratio (r = – 0.682, p < 0.001). There was no significant correlation of estrogen, testosterone, or E2/T ratio to lipid profile (total cholesterol, HDL, LDL) in women with CAD.
Conclusion E2/T ratio was significantly lowered in postmenopausal women with CAD. E2/T ratio may be a used a predictor of CAD in postmenopausal women
Keywords
sex hormones
postmenopausal women
coronary artery disease
-
Abstract Image
Abstract Image
Introduction
Incidence of coronary artery disease (CAD) in women increases steeply after menopause.1 An important factor for the increased CAD postmenopause may be imbalance of sex steroid hormones.2 3 Earlier studies in evaluating the association between sex hormones in postmenopausal women and CAD development have shown conflicting results. Few studies showed a negative relationship between serum estrogen and CAD, and a positive relationship between serum-free testosterone and the degree of CAD in postmenopausal women.4 5 However, Rexrode et al and Barrett et al showed no association between estrogen levels in CAD development.6 7 In addition to sex hormones, low levels of sex hormone-binding globulin (SHBG), hyperinsulinemia, and decreased dehydroepiandrosterone sulfate (DHEA-S) levels have also been associated with increased risk of CAD development.8 9 Due to conflicting results and limited data from the Indian subcontinent, we investigate the association between sex hormones and CAD development. The aim of this study was to measure the levels of serum sex hormones in postmenopausal women undergoing coronary angiography and to observe the association between an imbalance in the endogenous sex hormones and the presence of CAD.
Methods
Postmenopausal women undergoing coronary angiography from May 2015 to June 2016 for suspected CAD were recruited from the department of cardiology at our institute. Detailed medical history was recorded. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (metre2). Conventional risk factors for CAD were evaluated. Subjects taking estrogen, thyroid hormone, insulin, or any other hormone and digitalis (which has been reported to affect the estradiol and testosterone levels) were excluded from the study. Patients with serious illness, underlying malignancy, and ovariectomy history were also excluded from this study. All subjects gave informed consent. 2D echocardiography was performed in all the subjects prior to coronary angiography. Patients according to left ventricular ejection fraction (LVEF) were divided into normal, mild, moderate, and severe left ventricle dysfunction (LVD) with LVEF of ≥ 55%, 45 to 54%, 30 to 44%, and ≤ 30%, respectively. Coronary angiography was performed via radial/femoral route, and angiograms were taken with multiple views. Individuals with ≥ 50% stenosis of at least one major coronary artery were defined as CAD cases, and those with normal coronary arteries constituted the non-CAD (control) group.
Hormonal Analysis
Morning blood samples (at least 8 hour fasting) were taken for hormonal analysis and lipid profile prior to coronary angiography. Serum estradiol, testosterone, SHBG, insulin, and DHEA-S were measured. Estradiol, SHBG, and insulin were analyzed by chemiluminescence immunoassay (Siemens IMMULITE 1000 Chemiluminescent Technology), and testosterone and DHEA-S were analyzed by radioimmunoassay (Immunotech Beckman-Coulter RIA KIT). Hormone assays (estradiol, testosterone, DHEA-S, SHBG, insulin) were performed at the core laboratory in our institute from stored serum samples.
Statistical Analysis
Statistical analysis was done using IBM SPSS Statistical Software (IBM SPSS Statistics version 20.0, IBM SPSS, USA). Variables were reported as means ± standard deviation (SD). The skewed variable (SHBG) was logarithmically transformed. Variables between the control and case groups were compared using independent t-tests. The multiple linear regression analysis was used to assess the association between sex hormones and lipid parameters. In addition, the Pearson correlation between estradiol, testosterone, SHBG, DHEA-S, and insulin was estimated in postmenopausal women. A p-value of 0.05 or less was taken as significant.
Results
Basic Demographic Profile (Table 1)
|
Total n = 121(%) |
CAD group n = 69(%) |
Non-CAD group n = 52(%) |
p-Value |
|
|---|---|---|---|---|
|
Mean age (years) |
58.8 ± 8.7 |
58.4 ± 9.8 |
59.3 ± 7.1 |
0.571 |
|
Height (cm) |
151.8 ± 5.8 |
152.8 ± 5.8 |
150.5 ± 5.3 |
0.126 |
|
Weight (kg) |
60.2 ± 7.6 |
61.1 ± 8.6 |
58.9 ± 5.7 |
0.124 |
|
BMI (kg/mt2) |
26.2 ± 3.5 |
26.0 ± 2.1 |
26.1 ± 3.0 |
0.776 |
|
Diabetes |
42(34.7%) |
27 (39.1%) |
1528.8%) |
0.239 |
|
Hypertension |
67(55.4%) |
41 (59.4%) |
26(50%) |
0.302 |
|
Smoking |
0 |
0 |
0 |
|
|
Family h/o CAD |
6(5%) |
3 (4.3%) |
3 (5.8%) |
0.518 |
|
TC (mg/dl) |
132.2 ± 26.4 |
136.0 ± 28.7 |
127.0 ± 22.0 |
0.061 |
|
Triglyceride(mg/dl) |
135.4 ± 58.4 |
137.7 ± 70.5 |
132.4 ± 37.3 |
0.627 |
|
LDL (mg/dl) |
82.2 ± 25.6 |
82.8 ± 31.6 |
81.4 ± 13.4 |
0.769 |
|
HDL (mg/dl) |
35.4 ± 9.8 |
34.9 ± 9.7 |
36.1 ± 9.9 |
0.496 |
|
HDL:LDL ratio |
0.47 ± 0.21 |
0.48 ± 0.25 |
0.46 ± 0.2 |
0.555 |
|
LVEF |
||||
|
Normal |
86 (71.1%) |
40 (58%) |
46 (88.5%) |
|
|
Mild LVD |
27 (22.3%) |
25 (36.2%) |
2 (3.8%) |
|
|
Moderate LVD |
4 (3.3%) |
4 (5.8%) |
– |
|
|
Severe LVD |
4 (3.3% |
– |
4 (7.7%) |
|
|
Coronary angiography |
||||
|
Normal |
52 (43%) |
– |
52 (100%) |
|
|
SVD |
38 (31.4%) |
38 (55.1%) |
– |
|
|
DVD |
17 (14%) |
17 (24.6%) |
– |
|
|
TVD |
14 (11.6%) |
14 (20.3%) |
– |
Abbreviations: BMI, body mass index; DVD, double-vessel disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVD, left ventricular dysfunction; LVEF, left ventricular ejection fraction; SVD, single-vessel disease; TC, total cholesterol; TVD, triple-vessel disease.
Demographics and baseline characteristics for the total population and CAD and non-CAD groups are summarized in Table 1. A total of 121 postmenopausal females were included in the study, with the mean age of 58.8 ± 8.7 years. Out of 121, 69 women had CAD, and 52 were in the non-CAD group.
The most common clinical presentation among CAD patients was stable angina in 30 (43.5%) subjects, followed by ST-segment elevation myocardial infarction (STEMI) in 27 (39.1%). Unstable angina (UA) was diagnosed on admission in 7 (10.2%) CAD subjects, and 5 (7.2%) presented with non-ST-segment elevation myocardial infarction (NSTEMI). Among non-CAD females, the most common presentation was atypical chest pain; however, in view of the risk factors, coronary angiogram was done to rule out CAD. A total of 42 (34.7%) patients had diabetes and were on oral antidiabetic agents, while none were on insulin therapy. Risk factors diabetes, hypertension, and family history were similar in both CAD and non-CAD groups. Lipid profile (total cholesterol [TC], triglyceride [TGC], low-density lipoprotein cholesterol [LDL], high-density lipoprotein cholesterol [HDL], and HDL:LDL ratio) was similar among both groups. In CAD patients, the single-vessel disease (SVD) was present in 38 (55.1%) patients, whereas double-vessel disease (DVD)and triple-vessel disease (TVD) were present in 17 (24.6%) and 14 (20.3%) patients, respectively. Procedures were completed through radial route in 98 (91.6%) patients.
Hormonal Analysis (Table 2)
|
Total (n = 121) |
CAD (n = 69) |
Non-CAD (n = 52) |
p-Value |
|
|---|---|---|---|---|
|
Estradiol (pg/mL) |
31.2 ± 10.9 |
30.8 ± 12.0 |
31.6 ± 9.6 |
0.704 |
|
Testosterone (nmol/dl) |
12.8 ± 10.3 |
14.2 ± 10.5 |
10.9 ± 9.7 |
0.082 |
|
E2/T ratio |
4.4 ± 3.6 |
3.7 ± 2.6 |
5.4 ± 4.2 |
0.008 |
|
Log SHBG (nmol/l) |
1.52 ± 0.19 |
1.51 ± 0.19 |
1.54 ± 0.2 |
0.389 |
|
DHEA-S (mmol/l) a |
0.61 (0.16–5.99) |
0.45(0.16–5.99) |
0.62(0.16–2.85) |
0.823 |
|
Insulin (mIU/mL) |
8.84 ± 6.76 |
9.23 ± 6.68 |
8.32 ± 6.9 |
0.465 |
Abbreviations: DHEA, dehydroepiandrosterone sulfate; E2/T ratio, estradiol/testosterone ratio; SHBG, sex hormone-binding globulin.
Postmenopausal women with CAD had nonsignificantly lower serum estradiol levels (30.85 ± 12.01 pg/mL vs. 31.61 ± 9.57 pg/mL, p = 0.704) and higher serum testosterone levels (14.2 ± 10.5 nmol/dl vs. 10.9 ± 9.7 nmol/dl, p = 0.08), respectively, as compared with women with nonsignificant CAD. However, the estradiol/testosterone (E2/T) ratio was significantly lower in postmenopausal women with CAD (3.7 ± 2.6 vs. 5.4 ± 4.2, respectively, p = 0.008). SHBG levels were found similar in postmenopausal women with CAD and non-CAD women (1.51 ± 0.19 nmol/l vs. 1.54 ± 0.2 nmol/l, p = 0.389). DHEA-S and insulin levels were not significantly different between the two groups. Correlation of sex hormones with lipid levels (Table 3).
|
Sex hormone |
TC |
TGC |
HDL |
LDL |
HDL:LDL ratio |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Β coefficient |
p-Value |
Β coefficient |
p-Value |
Β coefficient |
p-Value |
Β coefficient |
p-Value |
Β coefficient |
p-Value |
|
|
S. estradiol |
– 0.04 |
0.80 |
–0.19 |
0.20 |
–0.10 |
0.50 |
0.07 |
0.62 |
–0.10 |
0.48 |
|
Testosterone |
0.16 |
0.47 |
0.24 |
0.23 |
–0.19 |
0.36 |
0.15 |
0.47 |
–0.27 |
0.20 |
|
E2/T ratio |
– 0.03 |
0.89 |
0.48 |
0.06 |
–0.02 |
0.93 |
–0.01 |
0.95 |
–0.08 |
0.70 |
|
Log SHBG |
– 0.09 |
0.46 |
–0.14 |
0.22 |
0.12 |
0.35 |
–0.17 |
0.17 |
0.22 |
0.08 |
|
DHEA-S |
0.05 |
0.69 |
–0.11 |
0.38 |
0.14 |
0.26 |
0.09 |
0.46 |
0.01 |
0.99 |
|
Insulin |
–0.01 |
0.89 |
0.17 |
0.15 |
0.22 |
0.09 |
–0.17 |
0.19 |
0.22 |
0.08 |
Abbreviations: DHEA-S, dehydroepiandrosterone sulfate; E2/T ratio, estradiol/testosterone ratio; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SHBG, sex hormone-binding globulin; TC, total cholesterol; TGC, triglycerides.
Multiple linear regression analysis between sex hormones and lipid parameters showed no significant correlation of estrogen, testosterone, or E/T ratio to lipid parameters (TC, HDL, LDL, and HDL:LDL ratio). E2/T ratio was negatively correlated with TC, LDL, and HDL but positively correlated with TGC level, but the correlation was nonsignificant. Among postmenopausal females, the serum level of estradiol predicted the E2/T ratio (r = 0.316, p < 0.001) and it was positively associated with DHEA-S (r = 0.181, p = 0.047). Testosterone was negatively associated with E2/T ratio (r = – 0.682, p < 0.001). There was no significant association between SHBG, DHEA-S, and insulin levels with E2 /T ratio. DHEA-S levels were positively correlated with estrogen levels in postmenopausal females (r = 0.240, p 0.047) (Table 4). No significant association was found between the level of sex hormones and the extent of CAD as assessed by the number of vessels involved (SVD, DVD, TVD). There was no correlation between the number of years of menopause attained and E2/T ratio.
|
Estradiol |
Testosterone |
E2/T ratio |
SHBG |
DHEA-S |
||
|---|---|---|---|---|---|---|
|
Testosterone |
Pearson correlation |
0.139 |
||||
|
p-Value |
0.256 |
|||||
|
E2/T ratio |
Pearson correlation |
0.238 |
–0.724 |
|||
|
p-Value |
0.047 |
< 0.001 |
||||
|
SHBG |
Pearson correlation |
– 0.083 |
0.042 |
–0.005 |
||
|
p-Value |
0.503 |
0.735 |
0.966 |
|||
|
DHEA |
Pearson correlation |
0.240 |
0.212 |
–0.113 |
0.004 |
|
|
p-Value |
0.047 |
0.080 |
0.353 |
0.973 |
||
|
Insulin |
Pearson correlation |
– 0.158 |
0.136 |
–0.156 |
–0.092 |
0.074 |
|
p-Value |
0.195 |
0.266 |
0.200 |
0.457 |
0.547 |
Abbreviations: DHEA-S, dehydroepiandrosterone sulfate; E2/T ratio, estradiol/testosterone ratio; SHBG, sex hormone-binding globulin.
Discussion
The present study revealed a significantly lower E2/T ratio in postmenopausal women with CAD than non-CAD postmenopausal women (normal coronaries in angiography). The study by Dai et al also showed E2/T ratio was significantly lower in women with CAD as compared with non-CAD and an imbalance of E2/T ratio showed a strong association with cardiovascular risk factors in postmenopausal women with CAD.10 Pathogenesis of association between sex hormones and an enhanced risk of CAD has been hypothesized by various studies.11 12 13 14 In addition to having a favorable effect on lipid profile, estrogen also affects vascular tone, leading to vasodilatation. Estrogen and testosterone have effect on the vascular tone and endothelial function in the vascular system. These changes may alter vasculature and enhance atherogenicity in vessels, which may predispose to CAD. E2/T ratio is now increasingly being studied for association with CAD, as it shows the effect of both estrogen and testosterone on the vasculature. In a recent prospective study, Zhao et al followed-up 2,834 postmenopausal women participating in the multiethnic study of atherosclerosis (MESA) for 12 years.15 The relationship of endogenous sex hormone levels with incident cardiovascular disease (CVD), CAD, and heart failure at the end of 12 years were assessed. A higher T/E2 ratio was associated with an increased incidence of coronary heart disease (CHD) and heart failure events. Similar findings also revealed in our study that E2/T ratio was significantly lower in postmenopausal women with CAD (p = 0.008), suggesting that the E2/T ratio may be a more reliable parameter in assessing risk for CAD.
Despite the pathological hypothesis of sex hormones with CAD development, various prospective studies have yielded conflicting results. A study by Barrett et al in 651 postmenopausal women with 19-year follow-up showed lower baseline levels of total testosterone were not associated with increased CVD events, which is similar to our study's findings.7 The role of DHEA-S and SHBG in the development of CAD is also controversial. A study by the WISE group showed lower DHEA-S levels were linked with higher CVD mortality and all-cause mortality.16 In our study, no association was found between DHEA and SHBG levels, similar to earlier studies.6 16
Endogenous sex hormones also affect lipid metabolism. A study conducted by Haffner et al in 253 postmenopausal women showed a positive correlation of total testosterone with TC, while free testosterone-related inversely to HDL-cholesterol.17 Free androgenic index (FAI) was positively associated with LDL-cholesterol but not HDL-cholesterol or TGC in a study by Shelly et al.18 On the contrary, few studies found no association between endogenous testosterone or SHBG and lipid profile in postmenopausal women.9 19 Our study also showed no definite correlation of estrogen, testosterone, or E2/T ratio to lipid profile (TC, HDL, LDL) in postmenopausal women with CAD.
Based on the above data, we can consider that it is not only the level of either estrogen or testosterone alone but also the imbalance of both hormones responsible for the development of CAD. Although there are trends toward lower estrogen and higher testosterone levels in CAD patients in postmenopausal women, it is the E2/T ratio that is more predictable of CAD development in women rather than the absolute value of estrogen or testosterone.
Limitation
The first limitation is the small sample size, which may be why we could not completely evaluate the relation of sex hormones with different risk factors of CAD and the interrelation of these hormones. Second, patients who were already on statins were not excluded; hence, correlation of hormones with lipid profile could not be elicited de novo in this study.
Conclusion
To conclude, the present study shows that imbalance of estrogen and testosterone associated with atherosclerosis and coronary artery disease in postmenopausal women and estradiol/testosterone (E2/T) ratio may be used as a predictor of coronary artery disease in these patients.
Conflict of Interest
There is no relationship relevant to the contents of this paper to disclose and no conflict of interest.
Funding No grant, contracts, or other form of financial support has been taken.
References
- Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol Metab Clin North Am. 2013;42(02):227-253.
- [Google Scholar]
- Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224(01):228-234.
- [Google Scholar]
- Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(02):210-241.
- [Google Scholar]
- Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidemiol. 2001;154(06):489-494.
- [Google Scholar]
- Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 1997;17(04):695-701.
- [Google Scholar]
- Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108(14):1688-1693.
- [Google Scholar]
- Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ. 1995;311:1193-1196. (7014):
- [Google Scholar]
- Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111(10):1242-1249.
- [Google Scholar]
- Hyperinsulinaemia and decreased plasma levels of dehydroepiandrosterone sulfate in premenopausal women with coronary heart disease. J Intern Med. 1995;237(05):465-472.
- [Google Scholar]
- Estradiol/Testosterone imbalance: impact on coronary heart disease risk factors in postmenopausal women. Cardiology. 2012;121(04):249-254.
- [Google Scholar]
- Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91(11):2742-2747.
- [Google Scholar]
- Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig. 2014;18(02):89-103.
- [Google Scholar]
- Local oestrogenic/androgenic balance in the cerebral vasculature. Acta Physiol (Oxf). 2011;203(01):181-186.
- [Google Scholar]
- Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71(22):2555-2566.
- [Google Scholar]
- DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health–National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischemia Syndrome Evaluation (WISE) J Clin Endocrinol Metab. 2010;95(11):4985-4992.
- [Google Scholar]
- Relation of sex hormones and dehydroepiandrosterone sulfate (DHEA-SO4) to cardiovascular risk factors in postmenopausal women. Am J Epidemiol. 1995;142(09):925-934.
- [Google Scholar]
- Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann Epidemiol. 1998;8(01):39-45.
- [Google Scholar]
- The association of androgenic sex steroids with serum lipid levels in postmenopausal women. Acta Obstet Gynecol Scand. 2004;83(05):487-490.
- [Google Scholar]







