Translate this page into:
Right Heart Catheterization-Revisited
*Corresponding author: J. Cecily Mary Majella, Department of Cardiology, Tamil Nadu Government Multi Super Speciality Hospital, Chennai, Tamil Nadu, India. drmajella@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Cecily Majella J, Chhabra S. Right Heart Catheterization-Revisited. Indian J Cardiovasc Dis Women. 2024;9:28-34. doi: 10.25259/IJCDW_14_2023
Abstract
Right heart catheterisation is a useful technique to diagnose - Pulmonary artery hypertension (PAHT), differentiate constrictive/restrictive physiology, and to monitor vasodilator treatment outcomes in PAHT. Helpful to assess and quantify intracardiac shunts. To assess the hemodynamics and fluid management after surgical procedures, myocardial infarction with complications, cardiac failure, and cardiogenic shock. It is a very useful method for pre cardiac transplant evaluation, to assess cardiac transplant recipients' surveillance status. Pre and post procedure evaluation of left ventricular assist devices (LVAD).
Keywords
Cardiac index
Pulmonary artery hypertension
Pulmonary artery pulsatility index
Right heart catheterization
INTRODUCTION
In 1929, German physician Werner Forssmann inserted a 65-cm urethral catheter to execute the first successful right heart catheterization. With their ground breaking research, Cournand and Dickinson won Nobel Prize in 1956 for the field of Medicine.[1]
Dr. Swan inflated a balloon at the end of the pulmonary catheter to take instantaneous readings of the right atrial and pulmonary arterial pressure. Due to Dr. Ganz’s advocacy of the thermodilution method and his novel addition of a thermistor to the tip, this indirect method of measuring cardiac output (CO) has become more accessible. Hence, the catheter to measure pulmonary arterial pressure was coined as “Swan-Ganz” catheter.[2,3]
RIGHT HEART CATHETERIZATION ACCESS
Access can be through the common femoral vein, internal jugular vein (IJV),[4] or the antecubital veins.[5] The IJV joins the subclavian vein to form the brachiocephalic vein. Brachiocephalic veins arising from both sides drain into superior vena cava (SVC), and right atrium (RA).[6] Shortest procedural time and lower incidence of hematomas have been reported with antecubital venous access.[7]
INDICATIONS
To diagnose Pulmonary artery hypertension (PAHT), constrictive/restrictive physiology, and heart failure with preserved ejection fraction
To monitor vasodilator treatment outcomes in PAHT
Cardiogenic shock
Cardiac tamponade
Quantification of intracardiac shunts
To assess the hemodynamics and fluid management after surgical procedures, myocardial infarction with complications, cardiac failure, and cardiogenic shock[8]
Congenital heart disease
Pre-cardiac transplant evaluation
To assess cardiac transplant recipients’ surveillance status
Acute onset or exacerbation of clinical symptomatology indicative of cardiac transplant rejection
Pre-procedure and post-procedure evaluation of left ventricular (LV) assist devices
When discrepancies are present between clinical signs and non-invasive assessment techniques.[9-13]
CONTRAINDICATIONS
Absolute contraindications
Infective endocarditis of tricuspid valve (TV) or pulmonary valves
Right heart thrombus or mass.
Relative contraindications
Hemorrhagic diathesis
It is necessary to take precautions in cases of arrhythmias and left bundle branch block (LBBB) to prevent triggering dysrhythmias.[14]
HARDWARES
To do thermodilution CO measurements, hardware catheters that contain a specialized thermistor are required to be used. The pulmonary artery catheter ranges from 5F to 8F and is 110 cm in total length. Each catheter has a distal port that is colored yellow, and a proximal port that is colored blue. The addition of a thermistor to the catheter results in the creation of a third port on the device.[11]
PROCEDURE
Under local anesthesia, venous access with or without ultrasound guidance is done. Femoral vein and IJV access can be made using an 18-gauge needle or a 21-gauge needle. In the antecubital vein, 21-gauge needle is preferable since it decreases the risk of injuring the arteries close to it. A sterile ultrasound probe sleeve is required if ultrasound guidance is required so as to avoid contamination of the sterile area. Through the venous access, an appropriately sized sheath is placed. The catheter is advanced to about 15 cm, and then only inflation of the balloon is done so as to prevent inflation of balloon within the sheath. The inflated balloon makes the catheter advancement easy to enter the right atrium (RA). From IJV to RA, the catheter will reach the RA in about 20 cm. Movements of catheters in fluoroscopy are also a helpful guide.
A pulsatile waveform of the RA is observed once RA is reached. Before recording the pressures, zeroing the system is mandatory. Zeroing involves opening the air-fluid transducer to air, and therefore, it is in equilibrium with the atmospheric pressure. The air-fluid transducer and heart should be in the same level. This is done by keeping the air-fluid transducer corresponding to the 4th intercostal space, at an imaginary plane linking anterior and posterior walls of the chest. If the transducer is at a lower level than the cardiac level, the resultant pressures may be spuriously higher. If the transducer is at a higher level, then the resultant pressures might be spuriously low.
The right atrial waveform is pulsatile as the catheter enters the RA. Zeroing the apparatus is required before taking pressure readings. Zeroing involves exposing the air-fluid transducer to air so that it equilibrates with atmospheric pressure. The air-fluid transducer needs to be placed at the heart’s level. The air-fluid transducer is held roughly at the level of the fourth intercostal space, at an imaginary plane between the anterior and posterior chest walls, to accomplish this. The pressures that are being observed could be exaggeratedly high if they are lower than the heart level. The pressures that are being detected could be spuriously low if the transducer is placed higher than the heart level.
After obtaining the RA pressure waveform, the catheter is adjusted to direct into the right ventricle, where the pressure is measured. The catheter is then typically wedged to determine the pulmonary capillary wedge pressure (PCWP). Then, the balloon after deflation is withdrawn a few centimeters to measure the pulmonary artery pressure (PAP). It is crucial that all the pressures are monitored at end-expiration only. Using distal yellow port, a sample of blood from the pulmonary artery is taken, and mixed venous oxygen saturation is measured. The Fick method must be used to obtain arterial saturation independently to calculate CO. The RA’s proximal blue port can be used to do thermodilution by injecting cold saline there, where it mixes with blood and the temperature difference is measured by a thermistor. To get an average CO and cardiac index (CI), thermodilution must be performed a minimum of 3 times. For the purpose of taking blood samples and calculating oxygen saturations, the catheter can be positioned in the SVC or inferior vena cava and also from RA and right ventricular (RV).[11,12]
The Utah-George Washington Diagnostic Algorithm[15] has moved right cardiac catheterization to the forefront and categorizes cases according to confirmed findings rather than conducting a panel of tests. The increased risk of having Group 2 pulmonary hypertension (PH) is so great that immediate treatment is essential without any additional diagnostic testing being performed. 18 Oncologists, however, require a definitive diagnosis that has been verified by a biopsy before beginning treatment because there are numerous treatment choices and various types of cancer [Figure 1].[15]
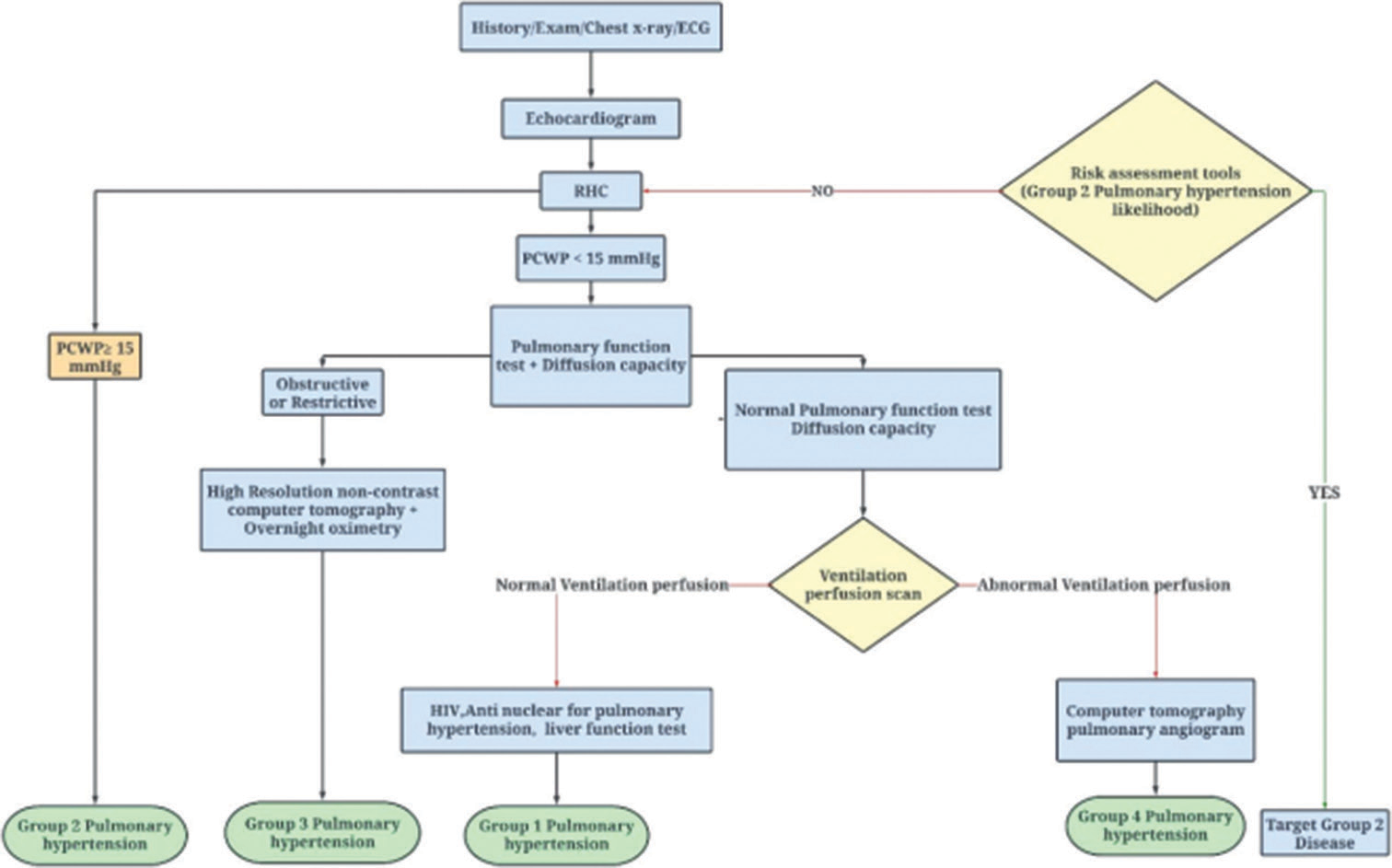
- UTAH-George Washington diagnostic algorithm. (PCWP: Pulmonary capillary wedge pressure, RHC: Right heart catheterisation, LVAD: Left ventricular assist device, ECG: Electrocardiogram.)
CLINICAL RELEVANCE
When a right cardiac catheterization is done, different pressure readings and hemodynamic data can be obtained. The range of the mean right atrial pressure (RAP) is 1–5 mm Hg. Systolic and diastolic pressures in the right ventricle should range from 15 to 30 mm Hg and 1 to 7 mm Hg, respectively. Systolic and diastolic PAP should be between 15 and 30 mm Hg and 4 and 12 mm Hg, respectively. The mean PAP is 15 mm Hg. 4–12 mm Hg is the range for normal PCWP. Calculations of CI, CO, pulmonary vascular resistance (PVR), systemic vascular resistance (SVR), right ventricle stroke work, pulmonary artery pulsatility index (PAPi), aortic and mitral valve areas using the Gorlin equation, and aortic valve area using the Hakki equation can all be done using the measured pressures [Table 1].
| Hemodynamic variables | Methods utilized for measurements | Normal values | Measurement in PAH | Recommendations |
|---|---|---|---|---|
| CO | Pulmonary blood flow measured: (1) Using the thermodilution principle (or 2) Fick method | 4–6 L/min | Normal or reduced | The favored technique is the thermodilution method but, the Fick method can be used. Oximetry should be performed in suspected left-to-right shunt and when pulmonary artery oxygen saturation is more than 75% |
| CI•min−1•m−2 | CI=CO/BSA | 2.4–4 | Normal or reduced | |
| PCWP mmHg | By measuring pressure waveforms | 4–12 | ≤15 | Balloon is Inflated in RA and advance catheter till it is wedged in the pulmonary artery. Do not do repeated successive inflations and deflations and remove the balloon when in inflation to avoid rupture of pulmonary artery. Mean of 3 end-expiratory pressure measurements are made to calculate PCWP |
| MAP (mmHg) | mPAP=Diastolic PAP+(Systolic–Diastolic PAP)/3 | Systolic: 15–25 Diastolic: 4–12 mPAP: 14±3 |
Mean PAP≥25 | |
| PVR wood units and PVRI wood units•m−2 | PVR=(mPAP–mean PAWP)/CO PVRI=PVR/BSA | ≤3 | PVR: >3 PVRI: ≥6 | For harmonization, PVR expressed in Wood units It may also be expressed as dyn•s−1•cm−5 (conversion: wood units×80) |
| RAP mmHg | Measured using pressure waveforms | 1–6 | Normal or increased | |
| Right ventricular pressure mmHg | Tracings to measure pressure waveforms | Systolic: 15–25 Diastolic 1–8 |
>30 Normal, or increased | |
| SVR wood units | SVR=(mSAP–RAP)/CO | 8.8–20 | PVR/SVR:<0.75 | Ratio of PVR to SVR>0.75 indicates significant pulmonary vascular disease |
| TPG mmHg | TPG=mPAP–PAWP | ≤12 | >12 | Helps to determine a pre-capillary component in post-capillary PH |
| DPG mmHg | DPG=Diastolic PAP–PAWP | <6 | >3 | Helps to determine pre-capillary component in post-capillary PH |
CO: Cardiac output, CI: Cardiac index, BSA: Body surface area, SVR: Systemic vascular resistance, PCWP: Pulmonary capillary wedge pressure, PAP: Pulmonary artery pressure, mPAP: Mean pulmonary arterial pressure, PH: Pulmonary hypertension, MAP: Mean arterial pressure, PVR: Pulmonary vascular resistance, PVRI: Pulmonary vascular resistance index, mSAP: Mean systolic arterial pressure, RAP: Right atrial pressure, PAWP: Pulmonary artery wedge pressure, TPG: Transpulmonary pressure gradient, DPG: Diastolic pressure gradient, RA: Right atrium, PAH: Pulmonary arterial hypertension
WAVEFORMS OF RA/PCWP
The waveform of the RAP shows two descents and three positive upstrokes. The first positive upstroke is the “a” wave, which stands for atrial systole. This is followed by the “x” descent, which indicates relaxation of the atrium. The following is the “c” wave, which is indicative of closure of TV. The subsequent positive wave is the “v” wave that displays passive filling of the atrium during contraction of RV. The “y” descent follows the “v” wave, which is due to emptying of atrium following the opening of the TV in the diastolic phase of the ventricle. The PCWP has three positive waves and two negative waves, just like the RAP waveform.
In atrial fibrillation, the “a” wave is absent because the atrial component to the waveform is lost. Tall “a” waves can be brought on by an increase in atrial pressure, which can [Table 1] occur in tricuspid stenosis. Cannon “a” waves are caused by atrioventricular dissociation, such as complete heart block (CHB), ventricular tachycardia (VT), or AV nodal re-entrant tachycardia (AVNRT). Large “v” waves are brought on by conditions that increase the volume of the ventricles during RV contraction, such as tricuspid regurgitation (TR) or mitral regurgitation (MR), right ventricular failure (RVF) or left ventricular failure (LVF), severe RV or LV non-compliance, and VSD. A “x” descent and rapid “y” descent are brought on by a rapid diastolic filling of the ventricle, which occurs in constrictive pericarditis (CP). The “y” descent is absent in cardiac tamponade because the diastolic pressures are equalized and the diastolic pressures in the ventricle do not decrease to the extent to permit full diastolic filling.
RIGHT VENTRICLE SYSTOLIC AND DIASTOLIC PRESSURES
When there is a pulmonary embolism or PH, the RV and pulmonary artery systolic pressure (PASP) are increased. Simultaneous right heart and left heart cardiac catheterization is the most effective way to detect ventricular interdependence in CP. Because there is no dependency in restricted cardiomyopathy, simultaneous measurements of the pressures in RV and LV agree.
The systolic area index, which can be calculated mathematically, is the ratio of the area of the right and left ventricles between inspiration and expiration. More than 95% sensitivity can be achieved in the detection of CP with a systolic area index higher than 1.1. Systolic area index seems to have the best predictive accuracy to distinguish CP and restrictive cardiomyopathy (RCM). Despite the fact that RCM and CP commonly manifest as chronic diseases, cardiac tamponade physiology is frequently acute.
HEMODYNAMICS OF CP
Constriction has an effect on hemodynamics by limiting the amount of blood that the heart can pump during diastole during the respiratory cycle while balancing the filling pressures on the right and left sides of the heart. High atrial driving pressures and unrestrained ventricular relaxation leads to increased initial rapid ventricular filling, which is followed by an abrupt rise in pressure from pericardial constraint. This explains the “square root” sign on the ventricular pressures and the sharp “y” descent on the atrial pressures pattern. Myocardial stretch during diastole is restricted by decreased pericardial compliance. Despite having high diastolic pressures, the low preload causes a reduced stroke volume. A prominent “x” drop is seen on atrial pressure tracings due to preserved atrial relaxation and an accentuated ventricular longitudinal contraction.
HEMODYNAMICS OF RCM
Rather than the intricate interplay of systemic and pulmonary pressures related with CP, RCM is due to intrinsic abnormalities of the myocardium that is unaltered during respiration. Similar to constriction, an early rapid filling of ventricles in the early part of the diastole, due to higher pressures in the atrium, the restrictive filling capacity of the myocardium which is stiff and unyielding, resulting in a pronounced “y” descent in the atrial, and the “square root” sign in the ventricular pressure curves, respectively. The stiffened ventricles are not able to receive further incremental volume during contraction of atrium, and therefore, the contribution due to atrial contraction is less. Unlike constrictive pathology, “x” descent is often blunted, due to inadequate relaxation of the atrium and limited descent of annulus toward apex. Since the non-compliant right ventricle cannot accommodate the raised venous return with inspiration, there is inspiratory diastolic flow reversal in the hepatic veins, whereas, in constriction, the hepatic vein flow reversal in diastole occurs in expiration. Unlike constriction, there are no discordances of intrathoracic/intracavitary pressures in restriction [Figures 2 and 3].
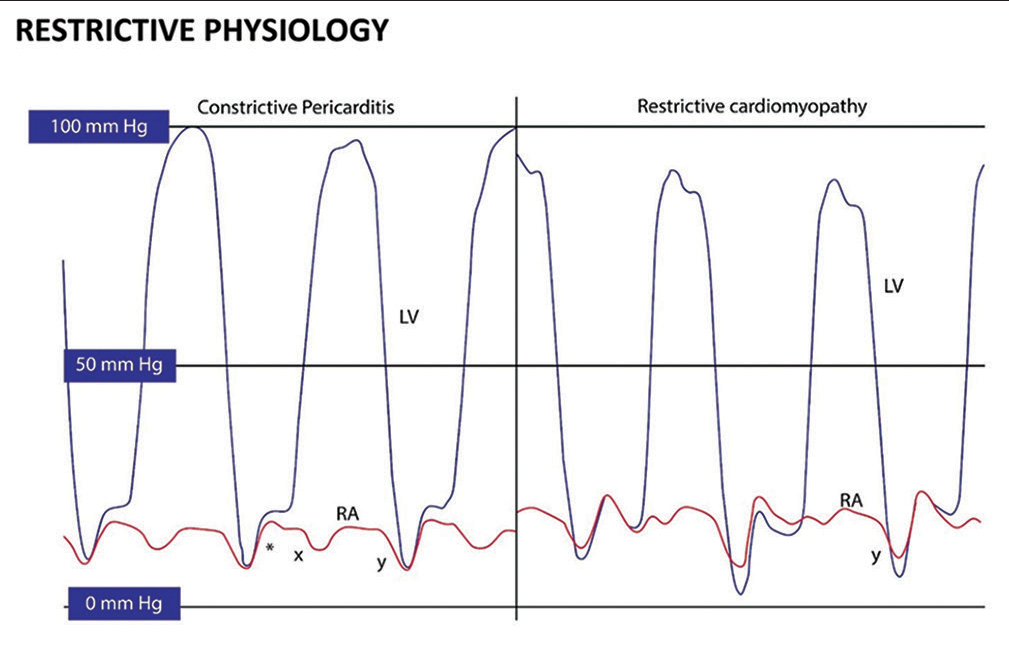
- (Left) left ventricular (LV)-dark and right atrium (RA)-red hemodynamics in constrictive pericarditis. “x” and “y” descents are prominent with a square root sign (*).
- (Right) LV-dark and RA-red hemodynamics in restrictive cardiomyopathy. A prominent “y” descent is present, but the “x” descent is blunted.
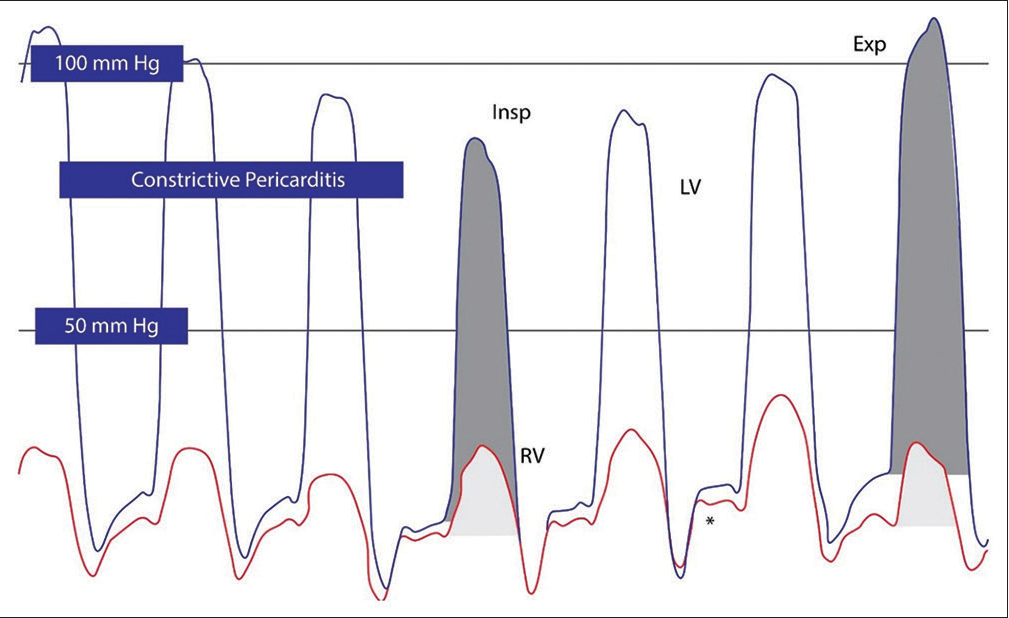
- Right ventricular (RV)/left ventricular (LV) hemodynamics in constriction and restriction (a) RV (Red), LV (Blue), and pressure tracings in constriction. Square root sign is seen in both pressure tracings (*). Prominent ventricular interdependence is seen by changes in systolic area index; RV (light gray) and LV (dark gray) areas under the curve are shown for inspiration/expiration. Increase in area of RV pressure curve during inspiration and reduction in the area of LV pressure curve in inspiration and vice versa in expiration in constrictive pericarditis. (b) LV and RV pressure tracings in restrictive cardiomyopathy. Although end-diastolic filling pressures are elevated and a square root sign (*) is present, there is no evidence of enhanced ventricular interdependence, with similar changes in LV and RV pressure curve areas. (Insp: Inspiration, Exp: Expiration).
CO
Right heart catheterization is commonly used to calculate CO using the thermodilution method and the indirect Fick principle. For this method, it is necessary to know the hemoglobin levels, central arterial and venous oxygen saturation levels, and maximal oxygen consumption values. According to the direct measurement approach, the maximum oxygen intake is calculated to be 250 mL/min or 125 mL/min. This is difficult and necessitates specialized equipment. Due to this, the indirect Fick approach – which depends on the assumed value of maximal oxygen consumption – is widely used. However, it has been shown that the CO determined using the indirect Fick methodology deviates from the direct Fick method by about 25% in up to 25% of individuals. Those with a BMI of over 40 kg/m appear to be particularly sensitive to these fluctuations.
10 cc of saline is delivered through the proximal blue port of the pulmonary artery catheter during the thermodilution technique. The temperature drop brought on by the mixture with right atrial blood is picked up by the thermistor at the catheter tip. However, there are problems with the thermodilution process. The thermodilution method is not regarded to be accurate when CO is low. In such situations, the area under the curve is small, and anything that alters the area under the curve further smaller will increase CO. When there is a recirculation of blood in severe TR or PR can lead to an inaccurate measurement of CO. It might erroneously overestimate CO in the presence of intracardiac shunts. It is crucial to be aware of the potential difficulties with these measures and the fact that the Fick and thermodilution procedures might result in inconsistent findings even within the same patient.
CI
The CI can be measured by correcting the CO for the total body surface area (BSA). The CO can be affected by factors such as the size and mass of the body. On the other hand, the CI of a healthy person is >2.5 L/min/square meter. Signs of cardiogenic shock include a PCWP >15 mm Hg and an CI lower than 2.2 L/min/square meter.
CARDIAC POWER INDEX
To calculate cardiac power output in Watts, multiply mean arterial pressure (MAP) into CO in liters per minute and divide by 451. Cardiac power index measures CO/BSA. Shock patients with cardiac power output below 0.6 Watts had high in-hospital mortality. Cardiogenic shock causes poor outcomes that are more precisely correlated with cardiac power output than with CI, EF, PASP, or MAP.[16]
PULMONARY AND SVR
SVR is calculated using the equation ([MAP–RAP] × 80/CO).
10–20 wood units, or 700–1600 dynes/s/cm, is the normal range.
PVR= ([Mean PAP–PCWP) × 80/CO).
Normal values – 20–120 dynes/s/cm, or <2 Wood units.
PAPI
PAPi is the ratio of PAP to RAP.[17,18] RAP is used to compute it (Systolic PAP–Diastolic PAP). PAPi of <0.9 demonstrates extraordinarily higher sensitivity and specificity for the prediction of the right heart failure and in-hospital mortality in acute inferior wall myocardial infarction.[17] PAPi <1.85 is helpful to assess if individuals may develop right heart failure and need assistance from RV hemodynamic devices following the implantation of LVAD. It is also a predictor of poor outcomes in subjects with chronic RVF.
COMPLICATIONS
Potential side effects include RBBB and ventricular arrhythmias, which are often temporary and gets resolved on removal or by correcting the position of the tip of the catheter. Rarely, third degree A-V blocks that necessitate the temporary implantation of pacemakers can occur in the presence of prior LBBB.
Air within the catheters or transducers may lead to air embolism. The patient may have abrupt onset of dyspnea, hypotension, tachycardia, and chest discomfort. In the event that air embolism is suspected, high-flow oxygen must be given while the patient is in the Trendelenburg position. This aids in lowering the blood’s nitrogen content and encourages the reabsorption of the air that was injected. Hyperbaric O2 is essential in specific circumstances.
Perforation of pulmonary arteries is rare and accounts for 0.03%, and it could happen when the balloon is inflated for an extended period of time and the catheter is inserted distally into the pulmonary arteries to measure the wedge pressure. This is especially true when there has been a history of PH and systemic anticoagulation. Sudden dyspnea and cardiogenic shock are two possible consequences. Fluoroscopy helps to determine if the catheter tip is very distal in the branches of the pulmonary artery. To reduce pulmonary hemorrhage if this transpires, the catheter must be left in situ with the balloon being inflated. To shield the unaffected side from the ongoing bleed, urgent intubation is done using a double-lumen endotracheal-tube and positioned in the lateral decubitus position with the affected side down. Embolization or emergency surgical intervention is considered if necessary. Potential issues with indwelling pulmonary artery catheters include infection of the access site, pulmonary infarction, perforation of the right-sided chambers or pulmonary artery, arrhythmias, and thrombosis of the access sites.
CONCLUSION
Cardiac catheterization still remains the standard of care for unraveling the mysteries of PH, illness severity assessment, prognosis assessment, and treatment response evaluation. This helps cardiologists provide optimum and early treatment for patients with PAHT with diverse etiologies.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- History of Right Heart Catheterization: 100 Years of Experimentation and Methodology Development. Cardiol Rev. 2010;18:94-101.
- [CrossRef] [PubMed] [Google Scholar]
- Catheterization of the Heart in Man with Use of a Flow-Directed Balloon-Tipped Catheter. N Engl J Med. 1970;283:447-51.
- [CrossRef] [PubMed] [Google Scholar]
- A Tale of Serendipity, Ingenuity, and Chance: 50th Anniversary of Creation of the Swan-Ganz Catheter. J Am Coll Cardiol. 2019;74:100-3.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy, Abdomen and Pelvis, Veins In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- [Google Scholar]
- Anatomy, Shoulder and Upper Limb, Veins In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- [Google Scholar]
- Anatomy, Head and neck, Internal Jugular Vein In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- [Google Scholar]
- Antecubital vs Femoral Venous Access for Right Heart Catheterization: Benefits of a Flashback. Can J Cardiol. 2015;31:1497.e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Value of Hemodynamic Monitoring in Patients with Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation. 2020;141:1184-97.
- [CrossRef] [PubMed] [Google Scholar]
- International Society of Heart and Lung Transplantation Position Statement on the Role of Right Heart Catheterization in the Management of Heart Transplant Recipients. J Heart Lung Transplant. 2019;38:235-8.
- [CrossRef] [PubMed] [Google Scholar]
- ACCF/SCAI/AATS/AHA/ASE/ASNC/ HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 Appropriate Use Criteria for Diagnostic Catheterization: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;59:1995-2027.
- [CrossRef] [PubMed] [Google Scholar]
- Right Heart Catheterisation: How To Do It. Heart Lung Circ. 2019;28:e71-8.
- [CrossRef] [PubMed] [Google Scholar]
- Images in Cardiovascular Medicine. Right Heart Catheterization, Coronary Angiography, and Percutaneous Coronary Intervention. Circulation. 2011;124:e428-33.
- [CrossRef] [Google Scholar]
- SCAI/HFSA Clinical Expert Consensus Document on the Use of Invasive Hemodynamics for the Diagnosis and Management of Cardiovascular Disease. Catheter Cardiovasc Interv. 2017;89:E233-47.
- [CrossRef] [Google Scholar]
- Videos in Clinical Medicine. Pulmonary-Artery Catheterization. N Engl J Med. 2013;369:e35.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive Prediction of Elevated Wedge Pressure in Pulmonary Hypertension Patients without Clear Signs of Left-Sided Heart Disease: External validation of the OPTICS risk score. J Am Heart Assoc. 2020;9:e015992.
- [CrossRef] [PubMed] [Google Scholar]
- SCAI Expert Consensus Statement: 2016 Best Practices in the Cardiac Catheterization Laboratory: (Endorsed by the Cardiological Society of India, and sociedad Latino Americana De Cardiologia Intervencionista Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne De Cardiologie d'intervention) Catheter Cardiovasc Interv. 2016;88:407-23.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary Artery Pulsatility Index is Associated with Right Ventricular Failure after Left Ventricular Assist Device Surgery. J Card Fail. 2016;22:110-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Impact Of Pulmonary Artery Pulsatility Index (PAPi) in Patients with Advanced Heart Failure: Insights from the ESCAPE Trial. J Card Fail. 2018;24:453-9.
- [CrossRef] [PubMed] [Google Scholar]







