Translate this page into:
Transcatheter interventions in refractory pulmonary artery hypertension and pulmonary embolism
*Corresponding author: J. Cecily Mary Majella, Chief Civil Surgeon, Senior Interventional Cardiologist, Department of Cardiology, Tamil Nadu Government Multi Super Speciality Hospital, Chennai, Tamil Nadu, India. drmajella@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chhabra S, Majella JC, Gupta A. Transcatheter interventions in refractory pulmonary artery hypertension and pulmonary embolism. Indian J Cardiovasc Dis Women 2023;8:52-7.
Abstract
Pulmonary artery hypertension causes remodeling of distal pulmonary arterial vasculature leading to increased resistance of the pulmonary arterial system, right ventricular dysfunction, and sudden cardiac death. The diagnosis of pulmonary arterial hypertension (PAH) diagnosis is made when mean pulmonary artery pressure during catheterization is ≥25 mmHg at rest, pulmonary vascular resistance (PVR) more than 3 wood units, a pulmonary capillary wedge pressure of <15 mmHg. One year survival rate is 86.3% and 5 year survival rate in PAH is 61.2%, and only 7 years of median survival. Although several breakthrough advances are made in the medical management for PAH, there are some patients who do not respond to medications and continue to detoriate despite optimal medical therapy. The non-responders to medical management are those patients whose right atrial pressure is >20 mmHg or cardiac index is <2.0 L/min/m2, which are pointers of poor prognosis. For medical refractory patients invasive procedures such as atrial septostomy, Potts shunt, and pulmonary artery denervation are a therapeutic or palliative strategy in the treatment of pulmonary artery hypertension and serve as a bridge before surgery and heart lung transplantation.
Keywords
Ballooon atrial septostomy
Potts shunt
Catheter directed thrombolysis
BALLOON ATRIAL SEPTOSTOMY
Creation of an atrial septostomy with a right-to-left shunt, is finally reserved for medically-refractory subjects who present with syncope and right ventricular (RV) failure atrial septostomy unloads the right ventricle, increases left ventricular (LV) preload, hence improves the cardiac output.[1] The concept behind doing atrial septostomy is based on the concept that pulmonary arterial hypertension (PAH) patients who have patent foramen ovale (PFO) with the right-to-left shunt survive longer than those without PFO and those with Eisenmenger’s syndrome who have alike mean Pulmonary artery pressure (PAP) and have decreased right atrial pressure (RAP),and less severe RV systolic dysfunction, and raised CO, and lower incidence of death rate in comparison to PAH subjects.[2] Large multicentre studies show ~86% of subjects undergoing balloon atrial septostomy (BAS) outlive the intervention and improved New York Heart Association (NYHA) functional status is observed in ~90% of patients.[1]
Transcatheter BAS is done through a gaurded IAS puncture using a Broken-Brough needle and by sequential dilation of the septal puncture using a noncompliant peripheral balloon. Oxygen saturation and LV end-diastolic pressure are measured sequentially with every stepwise increase in balloon diameter to prevent the decline in oxygen saturation by more than 10% or an increase in LVEDP >18 mmHg and thus precipitating pulmonary edema.[3-6] Case selection for atrial septostomy is critical because of the increased risk of periprocedural mortality; RAP >20 mmHg and a resting SpO2 of <90% in room air are important predictors of adverse events.[7] Post-procedure, patients have a drop in the right atrial pressure, increase in LA pressures, elevated CO, and a decline in SPO2 due to the shunting of blood that is deoxygenated. The improved hemodynamics after atrial septostomy has been well documented at rest and improves further with exercise. Atrial septostomy improves functional class, and 6-min walk study and a decline in brain-type natriuretic peptide values.[8,9]
Further research has thrown more light into the long-term outcomes of adult PAH patients who undergo atrial septostomy. In a single centre study comprising of 16 adult patients with pulmonary artery hypertension who were managed with prostanoids, 30 days and 1 year survival rates were 75% and 64%, respectively. In these subjects, mortality after atrial septostomy was due to failure to raise the cardiac output post procedure.[10] Balloon atrial septostomy outcomes as a standalone procedure are not as good as when the procedure is performed along with medications specific to reduce PAP in patients receiving specific drugs to reduce PAP. The median survival for subjects treated with atrial septostomy alone was 53 months in comparison to 83 months (P < 0.01) to subjects who underwent atrial septostomy in combination with PAH lowering drugs.[8]
Until now, few queries remain unanswered regarding benefits of BAS in PAH, which includes the ideal timing as to when the procedure has to be done, the exact sizing of the shunt, long-term sustainability, and when it is not used as a bridge to transplant as the shunt may tend to close spontaneously many months later in some patients.[1] The other transcatheter methods for atrial septostomy using cutting balloons, modified butterfly stents, fenestrated Amplatzer devices, and cryoablation, but these techniques have not been proven by studies to be superior to balloon septostomy.[11]
POTTS SHUNT
Potts shunt is an infra-tricuspid shunt that is created by anastomosing the left pulmonary artery (LPA) with descending aorta and off-load the right ventricle. The primary advantage of Potts shunt over balloon atrial septostomy is that the infra tricuspid shunt does not cause desaturation of arterial oxygen in upper half of body sparing both the neurological and coronary artery circulations. Although the vast majority of literature is with surgical Potts shunt created in children with PAH, recently percutaneous Potts shunt is created in adults.
Surgical Potts shunt was initially done successfully in two boys in 2004 with supra-systemic pulmonary artery hypertension, RV failure, and syncope. There was improvement in RV function and NYHA functional class but with development of polycythemia and lower limb cyanosis due to right-to-left shunt.[12] After a follow-up of nearly 2.1 years, all the survivors showed a significant improvement in the 6 min walk test, significant decrease in PAH reducing therapies and had near normal growth curves.[13]
Transcatheter Potts shunting was done in a small group of children, who had a tiny or probe-patent ductus arteriosus (PDA). The PDA was crossed using a wire from the pulmonary artery and bare metal stenting was done to keep it patent. A larger sized stent was placed so as to equalize the systolic pressures of pulmonary artery and systemic circulation. Post procedure echo demonstrated a large patent stent with non-restrictive flow and improved RV function.[13,14]
It was first in 2013, Esch et al. demonstrated transcatheter technique of Potts shunt in adults who were symptomatic and had medical-refractory pulmonary artery hypertension which portends risk of developing sudden cardiac death. Since PDA is mostly absent in adults the shunt is created retrogradely by creating a perforation in the descending aorta at a point where descending aorta is in close apposition with the LPA. The new tract between the LPA and the descending aorta is bridged using a covered stent (iCAST 7 × 22 mm) which serves as a functional shunt. The procedural success was three out of the four patients who underwent the procedure and one death due to haemothorax. Another patient died after 5 days the procedure which was attributed due to comorbidities. The remaining 2 patients were followed up for 4 and 10 months and both of them were alive and had symptomatic and functional class improvement.[15]
With the available limited experience, there is evidence that transcatheter procedure of Potts shunt in adults is still considered an experimental therapy, although one with promise. Future studies has to be directed towards optimal stent sizing, long-term patency of stent, and monitoring late complications if any. Modifications of surgical Potts shunt by inserting poly-tetra-fluoroethylene patch which allows transient unidirectional blood flow.[13,16] It is thus plausible that using a partially valved covered stent might also be helpful in such patients.
PULMONARY ARTERY DENERVATION (PADN-1)
Denervation of pulmonary artery is a technique in PAH based on the concept that PAP increases with pulmonary nerve stimulation.[17] Earlier studies showed that denervation of pulmonary artery with catheter ablation done <2 mm proximal to bifurcation of main pulmonary artery (MPA) had a significant decrease in pulmonary artery and RV pressures. These earlier studies, had shown short-term outcomes and histological analysis was not much convincing showing adequate denervation or nerve damage to explain the hemodynamic improvements.[18,19]
The first in man pilot study PADN-1 study (PADN-1 for treatment of PAH) which constituted twenty-one subjects with PAH (thirteen under treatment and eight controls) who were deemed unresponsive to medical management showed that the procedure resulted in clinical benefits. 13 patients for whom denervation pulmonary artery were at the MPA bifurcation and the ostium of right pulmonary artery and LPA by a radiofrequency (RF) ablation catheter using a temperature sensor (T0 of more than 50°C, energy of 10 watts, duration time of 60 s). Procedural success was defined as a decline in PAP of ≥10 mmHg without any procedural complications, and was present in 12 out of 13 subjects. In comparison to patients who did not give the consent for the intervention were kept as controls, and the follow-up at 3-months showed that patients who underwent denervation of pulmonary artery had a decline in mean PAPs and improved RV function and 6 min walk test.[20] The limitation of this study was that it was a non-randomized study, the lower risk group studied, and the lack of long term follow-up.
Recently 2 studies on denervation of pulmonary artery has shown promise in this technique. Chen et al. has now expanded the previous study and reported the hemodynamic outcomes of sixty six subjects with varying etiologies of pulmonary artery hypertension treated with denervation of pulmonary artery.[21] These subjects also underwent the same denervation protocols as used in the initial pilot study. The study also involved 39 subjects with the World Health Organization classification 1 pulmonary artery hypertension and twenty-seven subjects having pulmonary artery hypertension due to the left-sided heart diseases or due to chronic thromboembolic PAH. The absolute decline in mean PAP was 5 mmHg from baseline of 53.1 ± 19.1 mmHg immediately post procedure and further 6.6 mmHg decrease after 24 h. On a follow-up of 6 months, the mean PAP further reduced to 44.8 ± 16.4 mmHg which was statistically significant (P < 0.001) and persisted for upto 1 year. The improvements in hemodynamics were also associated with a decline in PVR and improvement in cardiac output. On 1 year review, a rise in PAH-related morbidity suggesting the progression of the disease in treated patients. Although the enlightens us widen the knowledge of pulmonary artery denervation in PAH, strong affirmations are limited because of the small study group, the heterogeneous nature of the study group, the non-randomized open label study design, and procedure being performed by only limited number of operators. This study however, provides the basis for a multicentric randomized clinical trial which will help to confirm if it is efficacious in the management of PAH.
Rothman et al. in his study demonstrated mechanistic insight of denervation of pulmonary artery procedure by showing the anatomical pattern of distribution of the nerves surrounding the pulmonary artery vasculature and the mode of nerve injury in a demonstrable porcine model of acute PAH.[22] They also documented that the distribution of nerves were circumferential with different vascular locations with respect to lumen of the vessel. Histology in the early post procedure demonstrated that after denervation of pulmonary artery the ablated lesions had disruption of intima and reduced medial thickness in the pulmonary arteries. S100 protein was reduced on vascular staining indicative of efficient nerve injury. It is now evident that if performed in large animal model, denervation of pulmonary artery results in ablation of the nerve and there is an acute reduction of PAPs and tone. However, long-term multicentre studies are required to assess the durability of this technique and validate if nerve regrowth will occur or not.[17]
INTERVENTIONAL TREATMENT OF PULMONARY EMBOLISM (PE)
The clinical presentation of acute PE might vary from asymptomatic to massive PE. More than 50% of pulmonary artery circulation is compromised in massive PE culminating into RV failure, circulation collapse, hypotension with or without shock and high mortality rates (up to 30% in untreated massive PE). Therapeutic measures in acute massive PE aim at rapid recanalization of pulmonary arteries, reduced RV afterload, reversal of RV failure and hemodynamic compromise, mitigate risk of recurrence, and chronic thromboembolic pulmonary hypertension [Figures 1-6].

- Catheters used for mechanical breakdown of thrombus.

- Total cut off of pulmonary artery.

- Mechanical breakdown and intrapulmonary thrombolysis (Arrow).

- Check pulmonary angio post intra pulmonary thrombolysis and infusion for 24 h.
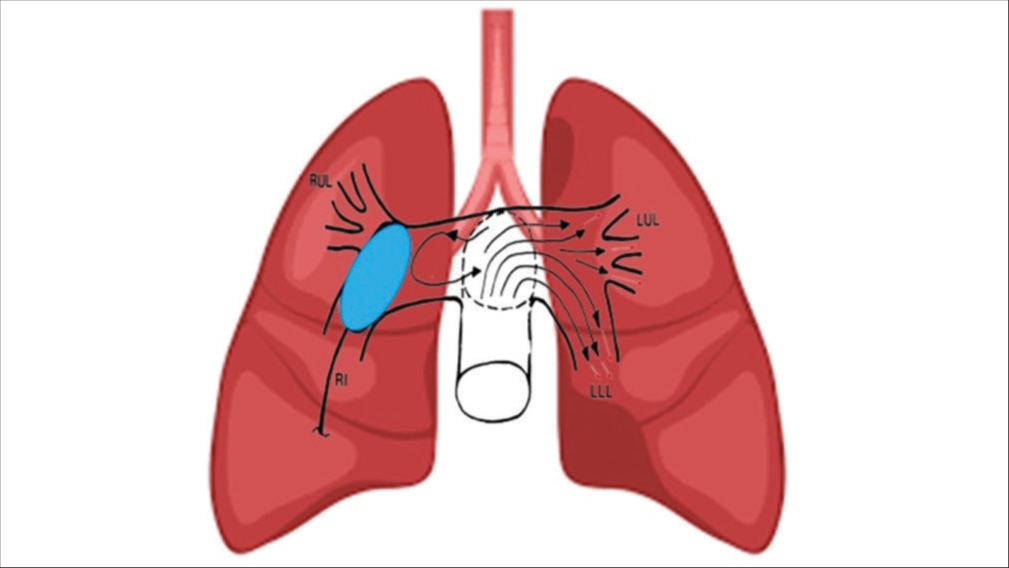
- Intrapulmonary local thrombolysis without mechanical breakdown and diversion of thrombolytic agent to opposite pulmonary tree.
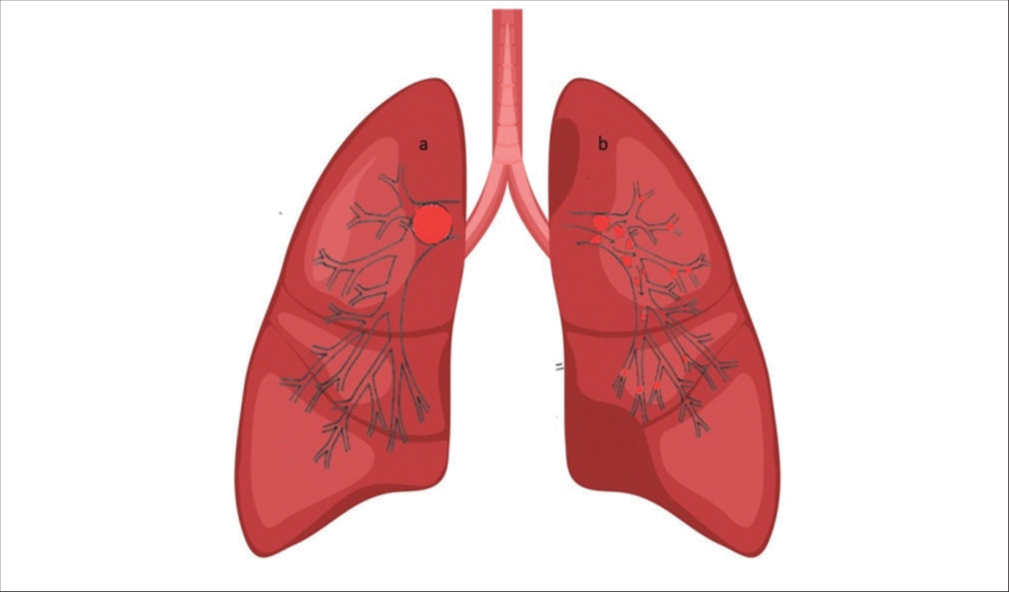
- Demonstration of the effect of mechanical breakdown of a completely occluding pulmonary artery embolus pre (a) and post (b) mechanical fragmentation and dispersal of the smaller thrombi in the peripheral pulmonary arterial tree and branches. Fragmentation and distal dispersion decrease paps and improve total pulmonary perfusion.
Systemic thrombolysis, the first line of defense in high risk massive PE with cardiogenic shock may not be feasible in substantial patients with contraindications and several patients might present with failed systemic thrombolysis. The expertise of surgical embolectomy in such emergent situations is limited to a few tertiary care centers. Percutaneous catheter embolectomy and mechanical breakdown of proximal pulmonary emboli followed by local intrapulmonary thrombolysis appear to be attractive options in this scenario given the relative ease to establish rapid pulmonary blood flow.
| Treatment | Advantages | Disadvantages |
|---|---|---|
| Systemic anticoagulation | Easy, Cost effective |
Treatment failure Time to effect |
| Systemic thrombolysis | Early reperfusion | Intracranial haemorrhage |
| Catheter directed lysis | Hybrid approach | Lack of RCT |
| Percutaneous thrombectomy | En bloc removal of thrombi | Expertise required, may not reach distal vessel |
| Surgical pulmonary embolectomy | Comprehensive proximal thrombectomy | Sternotomy, special expertise required |
| IVC Filters | Prevent further thrombus migration, avoid anticoagulation | Late mechanical complications because of failure to retrieve the filter |
Establishing flow of an occluded pulmonary artery by intervention has promising effect in day to day practice. Improved oxygenation after restoration of flow corrects the ventilation perfusion mismatch and reduces PVR.[23] This helps in reducing hemodynamic stress on the right ventricle and thus the preload to the left ventricle is maintained.[24]
Multiple possible interventions are available for acute PE:
Lower dose of thrombolytic therapy is required via catheter-directed thrombolysis (CDT) than systemic approach and it rapidly decreases PE thrombus burden.[25]
As suggested by SEATTLE II, CDT might be useful in intermediate-and high-risk PE patients.[26] Evaluation by Sardar et al. of initial SEATTLE II results found greater improvement following CDT in patients with high RV/ LV diameter ratio, higher mean pulmonary artery systolic pressure (PASP), and modified Miller index score. Patients with higher baseline heart rate had a lower reduction in RV/ LV diameter ratio, PASP. The Modified Miller index score documented significant improvement in patients with an abnormal baseline troponin and lesser in those with a higher body mass index and smokers.[27]
In a single center experience, 50 high risk PE patients were treated with standard pigtail catheter mechanical breakdown followed by local intrapulmonary thrombolytic therapy. Once flow across pulmonary artery was established with mechanical fragmentation of pulmonary thrombus by rotating 5F pigtail catheter; bolus dose of urokinase (4400 IU/kg) followed by infusion for 24 h was given in the thrombus. Antegrade pulmonary flow was restored in all patients with significant reduction in mean PAP, Miller score and Shock index from 41 ± 8 mmHg, 20 ± 5, 1.32 ± 0.3 to 24.52 ± 6.89, 5.35 ± 2.16, and 0.79 ± 0.21, respectively, (P <0.0001). One year follow-up recorded significant reduction in PASP.[28]
Besides, FLARE (FlowTriever Pulmonary Embolectomy Clinical Study), and EXTRACT-PE are landmark studies that highlighting the catheter-based therapies in acute PE.
6-min walk test has found to be feasible in assessing clinical outcome of patients undergoing CDT versus heparin.
CONCLUSION
Although catheter-based procedures are performed infrequently and are still evolving with time, it is becoming evident that transcatheter techniques and interventional cardiologists will play an important role in adults with PAH. Decision-making should be made by the multidisciplinary team with expertise and procedures performed in such centres with technical expertise. Catheter-based interventions either as a therapeutic or palliative procedure in PAH is an area of high innovation and is evolving day by day and future treatment would be the ideal combination of pharmacotherapy with novel transcatheter interventional techniques to improve clinical outcomes.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflict of interest
There is no conflict of interest.
Financial support and sponsorship
Nil.
References
- Atrial septostomy In: Voelkel NF, ed. Right Ventricle in Health and Disease. New York: Humana Press, Springer Science+Business Media; 2015. p. :419-37.
- [CrossRef] [Google Scholar]
- Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996;15:100-5.
- [Google Scholar]
- Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42-50.
- [CrossRef] [PubMed] [Google Scholar]
- Five-year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148:1043-54.
- [CrossRef] [PubMed] [Google Scholar]
- An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest. 2012;142:448-56.
- [CrossRef] [PubMed] [Google Scholar]
- Interventional and surgical therapeutic strategies for pulmonary arterial hypertension: Beyond palliative treatments. J Cardiol. 2015;66:304-14.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am J Cardiol. 1997;80:369-71.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J. 2011;38:1343-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of atrial septostomy on the concentration of brain-type natriuretic peptide in patients with idiopathic pulmonary arterial hypertension. Cardiol Young. 2007;17:5579.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon dilation atrial septostomy for advanced pulmonary hypertension in patients on prostanoid therapy. Catheter Cardiovasc Interv. 2015;85:1066-72.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary arterial hypertension in adults: Novel drugs and catheter ablation techniques show promise? Systematic review on pharmacotherapy and interventional strategies. Biomed Res Int. 2014;2014:743868.
- [CrossRef] [PubMed] [Google Scholar]
- Potts shunt in patients with pulmonary hypertension. N Engl J Med. 2004;350:623.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: Updated data from the first 24 patients. Eur J Cardiothorac Surg. 2015;47:e105-10.
- [CrossRef] [PubMed] [Google Scholar]
- Patent ductus arteriosus stenting (transcatheter Potts shunt) for palliation of suprasystemic pulmonary arterial hypertension: A case series. Circ Cardiovasc Interv. 2013;6:e18-20.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: Initial clinical experience. J Heart Lung Transplant. 2013;32:381-7.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical palliation of primary pulmonary arterial hypertension by a unidirectional valved Potts anastomosis in an animal model. J Thorac Cardiovasc Surg. 2011;142:1223-8.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging concepts in the molecular basis of pulmonary arterial hypertension: Part II: Neurohormonal signaling contributes to the pulmonary vascular and right ventricular pathophenotype of pulmonary arterial hypertension. Circulation. 2015;131:2079-91.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous pulmonary artery denervation completely abolishes experimental pulmonary arterial hypertension in vivo. EuroIntervention. 2013;9:269-76.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous interventional therapies for the treatment of patients with severe pulmonary hypertension. J Am Coll Cardiol. 2013;63:611-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary artery denervation to treat pulmonary arterial hypertension: The single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension) J Am Coll Cardiol. 2013;62:1092-100.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodynamic, functional, and clinical responses to pulmonary artery denervation in patients wtih pulmonary arterial hypertension of different etiologies: Phase II results from the PADN-1 study. Circ Cardiovasc Interv. 2015;8:e002837.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary artery denervation reduces pulmonary artery pressure and indices histological changes in an acute porcine model of pulmonary hypertension. Circ Cardiovasc Interv. 2015;8:e002569.
- [CrossRef] [PubMed] [Google Scholar]
- Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20:1314-31.
- [CrossRef] [PubMed] [Google Scholar]
- Improvements in pulmonary artery pressure and right ventricular function after ultrasound-accelerated catheter-directed thrombolysis for the treatment of pulmonary embolism. J Card Surg. 2014;29:455-63.
- [CrossRef] [PubMed] [Google Scholar]
- Catheter-based therapies in acute pulmonary embolism: The good, the bad, and the ugly. Circ Cardiovasc Interv. 2020;13:e009353.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of treatment response following ultrasound-facilitated catheter-directed thrombolysis for submassive and massive pulmonary embolism: A SEATTLE II Substudy. Circ Cardiovasc Interv. 2020;13:e008747.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of catheter fragmentation followed by local intrapulmonary thrombolysis in acute high risk pulmonary embolism as primary therapy. Indian Heart J. 2014;66:294-301.
- [CrossRef] [PubMed] [Google Scholar]







