Translate this page into:
Drug–Drug, Drug–Disease and Disease–Disease Interactions in COVID-19 with Cardiovascular Diseases (CVDs)
M. Jyotsna, MD, DM, FACC, FESC, FICC Department of Cardiology, Nizams Institute of Medical Sciences Punjagutta, Hyderabad 500082, Telangana India janaswamyjyotsna@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Coronaviruses are a large family of single positive-stranded, enveloped RNA viruses that can infect many animal species and humans. Human coronaviruses can be divided based on their pathogenicity. Globally so far, over nine million people have tested COVID-19 positive, of which, 4, 25,000 are in India. The FDA for the prevention or treatment of COVID-19 has approved no drugs or biologics. Numerous other antiviral agents, immunotherapies, and vaccines continue to be investigated and developed as potential therapies. Searching for effective therapies for COVID-19 infection is a complex process. The cardiovascular disease (CVD) drugs and the COVID-19 treating drugs show potent drug–drug interactions (DDI), disease–drug interactions, and disease–disease interactions.
Keywords
cardiovascular drugs
COVID-Drugs
interactions
pharmacotherapies
Introduction
Coronaviruses are a large family of single positive-stranded, enveloped RNA viruses that can infect many animal species and humans. Initially, from bat to human is the most likely model of cross-species transmission. Based on their pathogenicity, human Coronavirus can be divided into SARS-CoV, MERS-CoV, and the current novel SARS-CoV-2 which is of high pathogenicity.1
Globally so far, over nine million people have tested COVID-19 positive, of which, 4,25,000 are in India. It is an illness caused by a Coronavirus. The primary target of the Coronavirus disease (COVID-19) is the respiratory tract and that is why it is known as severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2). The FDA has approved no biologics or a drug for the treatment and prevention of COVID-19. Nevertheless, on May 1, 2020, FDA gave emergency authorization for the use of Remdesivir; severely affected patients have shown faster recovery based on preliminary hospital data. The search for effective treatment or therapies for COVID-19 infection is a complex and dynamic process. Many potential therapies like immunotherapies, antiviral agents, and vaccines continue to be developed and investigated. Pharmacotherapy guidelines and reviews have been published for COVID-19,2 including the global SOLIDARITY trail.3
The main point of this review is to discuss the drug-drug interactions (DDI) and drug-disease interactions, and we are not discussing whether the particular drugs are indicated or not.
Emergency Drugs Approved to Treat COVID-19/SARS-CoV-2
None of the drugs have proven to treat the COVID-19 infection effectively; however, clinical trials or studies are undergoing. Below are the few emergency drugs that have been approved by WHO, ICMR, FDA, etc. with some cautions.
1. Hydroxychloroquine–antimalarial drug
India first developed hydroxychloroquine (HCQ). On April 18, 1955, during World War II, FDA granted it approval for treating uncomplicated malarial infection, and autoimmune diseases like systemic lupus erythematosus (SLE), rheumatoid arthritis and chronic discoid lupus erythematosus (DLE).4
Mechanism: HCQ accumulates in individual organelle as well as in the parasite organelle lysosomes and affects the function by raising the pH. As a result of this, the virus (SARS-CoV & SARS-CoV-2) particles prevent fusion and entry into the cell. The SARS-CoV and SARS-CoV-2 target cell entry from angiotensin-converting enzyme 2 (ACE2) receptors. Terminal glycosylation of ACE2 receptors’ inhibition is done by HCQ. The spike protein of SARS-CoV-2 may less efficiently interact if the ACE2 is not in the glycosylated state; thus, HCQ inhibits further viral entry.3 5
2. Dexamethasone: anti-inflammatory drug.
According to RECOVERY trial,6 this is a lifesaving drug for moderate and severe COVID-19.
Mechanism: As the COVID-19 disease is partially due to direct viral effect or indirectly by cytokine or inflammatory storm, dexamethasone is a steroid, which mainly acts through the anti-inflammatory activity.
3. Azithromycin: antibacterial drug
In addition to antibacterial activity, it also has antiviral and immunomodulatory features.7 Caution to be taken when combined with HCQ.
4. Tocilizumab: immunosuppressive drug
This humanized IgG1 monoclonal antibody, Tocilizumab, was tested for COVID-19 in the TOCOVID-19 trail.8
Mechanism: It is a genetically engineered monoclonal antibody, humanized from a mouse antihuman IL-6R antibody, which mimics the natural antibodies against the microorganisms.
5. Remdesivir–antiviral drug
Drugs may be given to treat moderate-to-severe COVID-19 infected patients.
Mechanism: This drug stops the RNA polymerase enzyme, which is essential for viral replication.
6. Favipiravir–antiviral drug
This antiviral drug got approved for COVID-19 treatment in India, China, Russia, Italy, and Japan on June 11, 2020, but not in the US and EU.
Mechanism: It restricts transcription and replication of RNA-dependent RNA polymerase enzyme, which is essential for viral replication.9
COVID Drug-Drug Interactions (DDI)10
1. Azithromycin–HCQ
Macrolides are known to produce a prolongation of QT interval, which may precipitate the cardiac arrhythmias and tordaes pointes. If this drug is combined with HCQ, there are reports of QT interval prolongation in COVID-19 patients.11 In this current pandemic, HCQ or chloroquine in combination with a second-generation macrolide is being widely used for COVID-19 treatment in mild cases and as prophylactic. India moved out the drug for severe cases of treatment protocol, as an emergency use authorization was revoked by USFDA on June 15, 2020, and revised treatment guidelines were issued by ICMR.12 13
2. Azithromycin–Favipiravir, Remdesivir, Dexamethasone, Tocilizumab
No clinical interaction between these drugs is expected, depending on the metabolism, even though coadministration has not been studied.
3. HCQ–Remdesivir
In vitro, the antiviral activity of remdesivir was antagonistic by chloroquine in a dose-dependent manner, and there were decreasing levels of remdesivir triphosphate with increasing levels of chloroquine. However, this is not studied in humans. So, coadministration of HCQ with remdesivir is not recommended.14
4. HCQ–Tocilizumab
There are no coadministration studies with this combination. Nevertheless, interaction is likely, as HCQ is metabolized by CYPs 2C8, 3A4, and 2D6. Tocilizumab, per se, has no inhibitory or inducing effects on cytochromes directly, but indirectly it interacts through interleukins. Patients infected with COVID-19 may experience an elevation of IL-6, which has been shown to suppress expression/activity of CYP3A4, CYP2C19, CYP2C9, and CYP1A2. Tocilizumab will normalize cytochrome activity, as it is an inhibitor of IL-6. Because of this, close monitoring is required in this drug combination, even though drug dosage adjustment may not be required; however, different timing of drug administration may be essential if interact occurs.15
5. HCQ–Dexamethasone, Favipiravir
Clinically, no significant interaction.
6. Remdesivir–Dexamethasone, Favipiravir, Tocilizumab
Clinically no significant interaction.
7. Favipiravir–Dexamethasone, Remdesivir, Tocilizumab, Azithromycin, Hydroxychloroquine
Clinically, no significant interaction.
COVID-19 Drug–Cardiovascular (CVD) Drug Interactions10
The patients who already have cardiovascular disease (CVD) and those with CV risk factors are more prone to COVID-19 infection. Therefore, COVID-19 itself is known to produce CVD. The latest autopsy study16 and cardiac MRI studies showed that the incidence of myocardial injury was as high as 78%.17
So, DDI and drug–disease interactions are essential in this COVID-19 era.
Antiarrhythmics
Favipiravir, Remdesivir, Tocilizumab
Even though coadministration has not been studied, the metabolism and clearance of these drugs do not affect antiarrhythmic drugs. So, in warranted conditions, it is safe to combine them.
HCQ and Azithromycin
-
As HCQ and azithromycin are known to produce QT prolongation, better not combine with the antiarrhythmic drugs like amiodarone, which prolongs the QT.
-
HCQ increases propafenone concentrations due to inhibition of CP2D6 and leads to prolongation of the QT. Due to HCQ long half-life, QT prolongation persists long after the discontinuation of the drug.
-
HCQ or azithromycin and lidocaine coadministration are acceptable as the metabolic pathways for both drugs are different (CYP1A2 pathway for lidocaine and CYP3A4 for other drugs).
Anticoagulants, Antiplatelets, and Fibrinolytics
Favipiravir, Remdesivir, administered along with anticoagulants, antiplatelets, and fibrinolytics. No interactions expected even though studies are not done.
HCQ and Clopidogrel
This combination requires caution. Even though the initial metabolic activation pathway of clopidogrel does not interfere with the HCQ metabolic pathway, clopidogrel subsequently converted to a potent inhibitor of CYP2C8 causes an increase of HCQ exposure, as HCQ undergoes CYP-mediated metabolism by CYPs 2C8, 3A4 and 2D6.
However, HCQ is safe to combine with either heparin or warfarin.
Azithromycin and Warfarin
Even though the cause of prolongation of the prothrombin time (PT) when azithromycin is combined with coumarin-type oral anticoagulants is not known, the fact that it prolongs the PT requires monitoring.
Azithromycin, Aspirin (Antiplatelet), Clopidogrel, and Heparin
Coadministration may not lead to clinically significant interactions of these drug combinations.
Tocilizumab and Warfarin
CYP1A2, CYP3A4, and CYP2C9 metabolizer and S enantiomers of warfarin. Elevation of IL-6, due to COVID-19, causes suppression of expression/activity of CYP3A4, CYP2C19, CYP2C9, and CYP1A2. IL 6 inhibitor. For tocilizumab, when given with warfarin, international normalized ratio (INR) monitoring is recommended as the warfarin efficiency may decrease.
Tocilizumab and Clopidogrel
Like warfarin, the active metabolites of clopidogrel are via CYPs 3A4, 2B6, 2C19, and 1A2. So, with tocilizumab, the clopidogrel dose may need to be increased in order to maintain the therapeutic effect.
Dexamethasone and Warfarin
There are conflicting reports about this combination, so it is advisable to do coagulation indices to maintain the desired anticoagulant effect.
Converting warfarin to direct oral anticoagulant like apixaban is recommended with few exceptions, as monitoring of anticoagulant status is not required.
Hypertension/Heart Failure Agents
Favipiravir, Remdesivir, Tocilizumab Coadministration with Beta-blockers, calcium channel blockers, ACE inhibitors, diuretic, angiotensin receptor blockers (ARBs), nitrates
Clinically, no significant interaction.
HCQ, azithromycin and ACE inhibitors, diuretic, ARBs, nitrates
Clinically no significant interaction.
HCQ and beta-blockers
Coadministration of metoprolol and HCQ increased metoprolol AUC and Cmax by 72% and 65%, respectively.18 So, PR interval prolongation of metoprolol and QT prolongation of HCQ requires motoring in this combination.
HCQ and calcium channel blockers
Diltiazem can prolong the PR interval, and HCQ and azithromycin can prolong the QT interval. So, potential interactions may be seen.
HCQ, azithromycin, and ivabradine
Ivabradine causes bradycardia associated QT prolongation. So, it is recommended not to combine with drugs that cause QT prolongation, like HCQ and azithromycin.
HCQ, azithromycin, and digoxin
Renal transporters OATP4C1 and P-GP eliminate digoxin through the kidney. HCQ inhibits P-GP, so digoxin levels may increase. Besides, digoxin also can prolong the PR interval. When patients are receiving both HCQ and digoxin, serum digoxin levels should be closely monitored.
HCQ, azithromycin, and diuretics
Diuretics may produce hypokalemia, which requires monitoring when HCQ is coadministrated.
Dexamethasone and digoxin
This combination may cause an increased risk of digoxin-induced arrhythmias due to hypokalemia.
Lipid Lowering Agents
Favipiravir, Remdesivir, Tocilizumab, Hydroxychloroquine, Azithromycin, Dexamethasone, and lipid-lowering agents
Clinically, no significant interaction.
Lopinavir/Ritonavir
-
This drug is known to produce different AV blocks and QT prolongation. So, it is not to be combined with the drugs which prolong the QT.
-
Serum lipids may be increased.
-
They inhibit the CYP3A4 activity. This drug combination.
-
With statins, it increases the statin levels, so side effects of statins occur like rhabdomyolysis.
-
With clopidogrel or prasugrel, the serum concentration of active metabolites of these drugs decreases. So, the efficiency of these antiplatelets decreases.
-
With ticagrelor, it increases the serum concentration of active metabolites. So, the side effects of this antiplatelet drug increase.
-
With factor Xa inhibitors (apixaban and rivaroxaban) increase the concentration of these drugs, causing increasing bleeding risk.
-
However, according to Cao et al, there was no survival benefit treated with lopinavir/ritonavir in hospitalized adult patients with severe COVID-19.19
Ribavirin
This is an antiviral drug tried in COVID-19 in combination with other antiviral drugs.
-
It causes increase levels of lopinavir/ritonavir when given in combination.
-
It reduces the effect of warfarin.
In Table 1, the above said COVID-19 drug–CVD drug interactions were summarized. At the same time, few drugs need a dose, and adjustments are described in Table 2.
|
Drug |
CV drug class interactions |
CV adverse effects |
|---|---|---|
|
Abbreviations: CVD, cardiovascular disease. |
||
|
Remdesivir |
N/A |
Unknown |
|
Lopinavir/ritonavir |
Antiplatelets anticoagulants statin antiarrhythmics |
Different AV blocks; QT prolongation; increased serum cholesterol |
|
Chloroquine/hydroxychloroquine |
Antiarrhythmics |
AV block, bundle-branch block, QT prolongation, torsades de pointes |
|
Interferon-α, β |
Warfarin |
hypotension, arrhythmia, cardiomyopathy, myocardial infarction |
|
Methylprednisolone |
Warfarin |
Fluid retention, electrolyte disturbances, and hypertension |
|
Tocilizumab |
Antiplatelets anticoagulants statins evolocumab beta-blockers antiarrhythmics |
Hypertension Increased serum cholesterol |
|
Therapy |
Specific interaction |
MOA of drug interaction and specific dose adjustments |
Additional monitoring |
|---|---|---|---|
|
Abbreviations: DDI, drug–drug interaction; INR, international normalized ratio; HCQ, hydroxychloroquine. |
|||
|
Chloroquine/HCQ |
Beta-blockers |
Dose reduction of beta-blockers may be required |
|
|
QT-prolonging antiarrhythmics |
Further increases QT |
Monitor ECG |
|
|
Digoxin |
Dose reduction of digoxin may be needed |
Monitor serum digoxin levels |
|
|
Interferon-alpha, beta |
Warfarin |
Decreased dose may be needed |
Monitor INR |
|
Lopinavir/ritonavir |
Anticoagulants Direct factor Xa inhibitors Warfarin |
Increases the serum concentrations of direct factor Xa inhibitors May decrease serum concentration of warfarin |
Monitor INR with warfarin |
|
Statins |
Increases the concentration of statins |
Start at lowest possible dose statins and titrate up. |
|
|
QT-prolonging antiarrhythmics and digoxin Ranolazine Ivabradine |
More increase in QT |
Monitor ECG Monitor digoxin level |
|
|
Methylprednisolone |
Anticoagulants Warfarin |
Decreased dose may be needed |
Monitor INR |
|
Remdesivir |
N/A |
– |
N/A |
|
Tocilizumab |
Anticoagulants Direct factor Xa inhibitors Warfarin |
No dose adjustment recommendation |
Monitor INR |
|
Antiplatelets |
– |
||
|
Statins |
– |
||
|
Beta-blockers |
– |
||
|
Antiarrhythmics |
– |
||
COVID 19 Drugs and Cardiovascular Disease (CVD) Drugs Interactions
Not only does COVID-19 involve the cardiovascular system, the presence of CVD itself is a significant risk factor of COVID-19 disease development, which adds a bad prognosis also. Nearly one-fourth of patients with COVID-19 develop heart failure, and 7 to 33% of the deaths in COVID-19 were due to cardiogenic shock or arrest. It is essential to know the COVID-19 drug interaction in patients of CVD.
Potential Cardiac Side Effects of Current Medications Being Tried for COVID-19 Management
-
Methylprednisolone causes fluid retention, electrolyte derangement (especially hypokalemia), hypertension, and may increase viral shedding. Steroids should be avoided in patients of CVD with COVID-19 unless indicated as moderate-to-severe COVID disease.20
-
Interferon is an investigational drug for COVID-19 treatment. It affects the conduction system and is toxic to myocytes.21
-
Remdesivir, which is also an investigational drug, may produce hypotension and bradycardia in 1% of patients.22
-
Tocilizumab, even though known to increase cholesterol levels, is not yet known for long-term cardiac morbidity and mortality.23
Effect of CVD Drug in COVID-19
As the virus enters through the cell via ACE receptors, initially, there was concern about the usage of ACE and ARB’s role in increasing the proneness to develop the disease,24 as these drugs increase the ACE receptor levels further debated. Different hypertensive societies mentioned the safety and continuation of these drugs wherever it is indicated.25
Effect of COVID 19 ON CVD Disease
There is a separate review in the same issue mentioning the cardiovascular manifestations of COVID-19.
We are briefly discussing the impotence of COVID-19 in the management of CVD (Fig. 1) as COVID-19 imposes a significant burden on the diseased or compromises the CV system. COVID-19 causes profound systemic illness, impacting the CV system (Fig. 2). Rapid triage of non-COVID-19 CVD patients and healthcare workers’ (HCWs) safety is compromised.
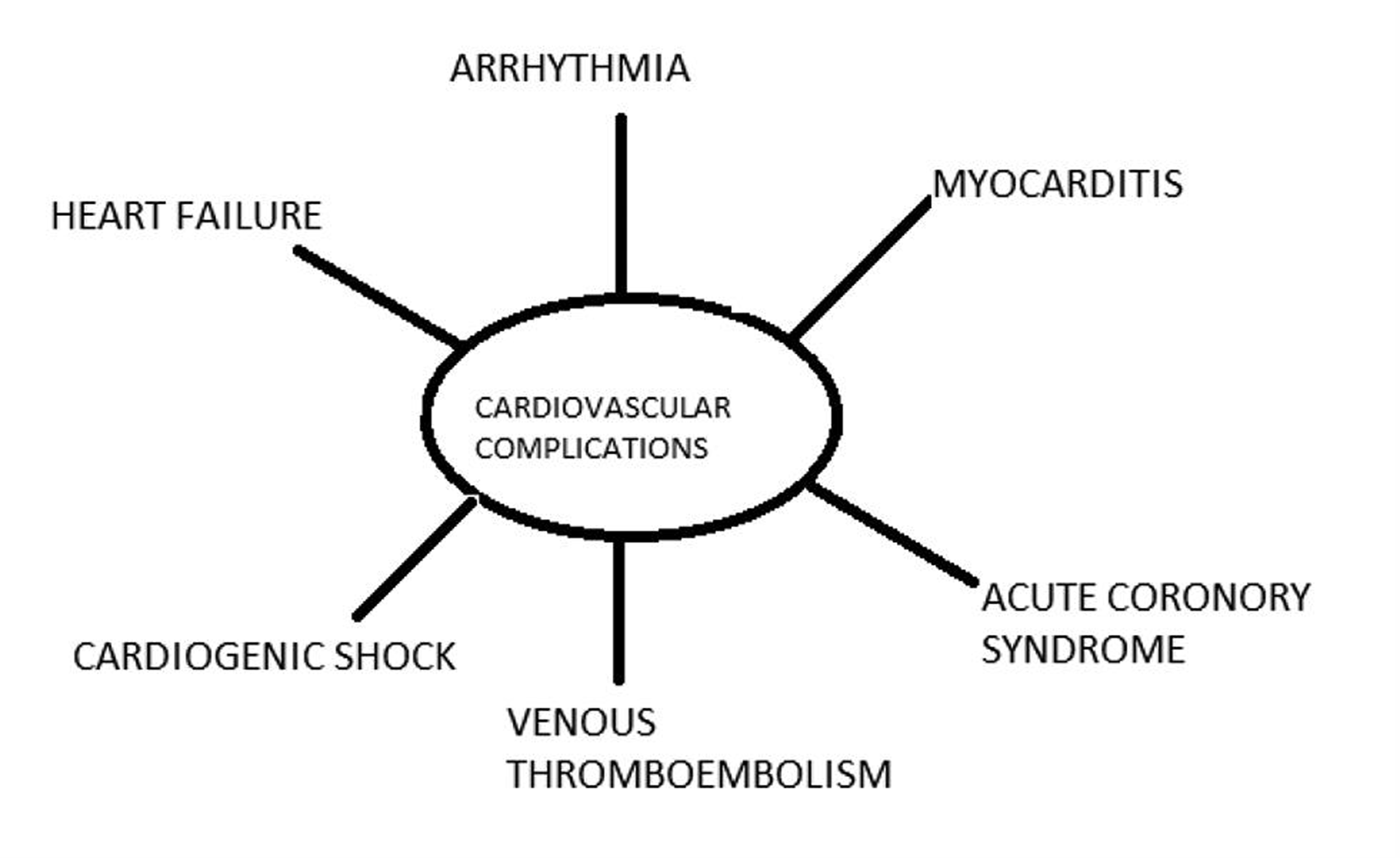
-
Fig. 1 Potential cardiovascular complications result from COVID-19.
Fig. 1 Potential cardiovascular complications result from COVID-19.
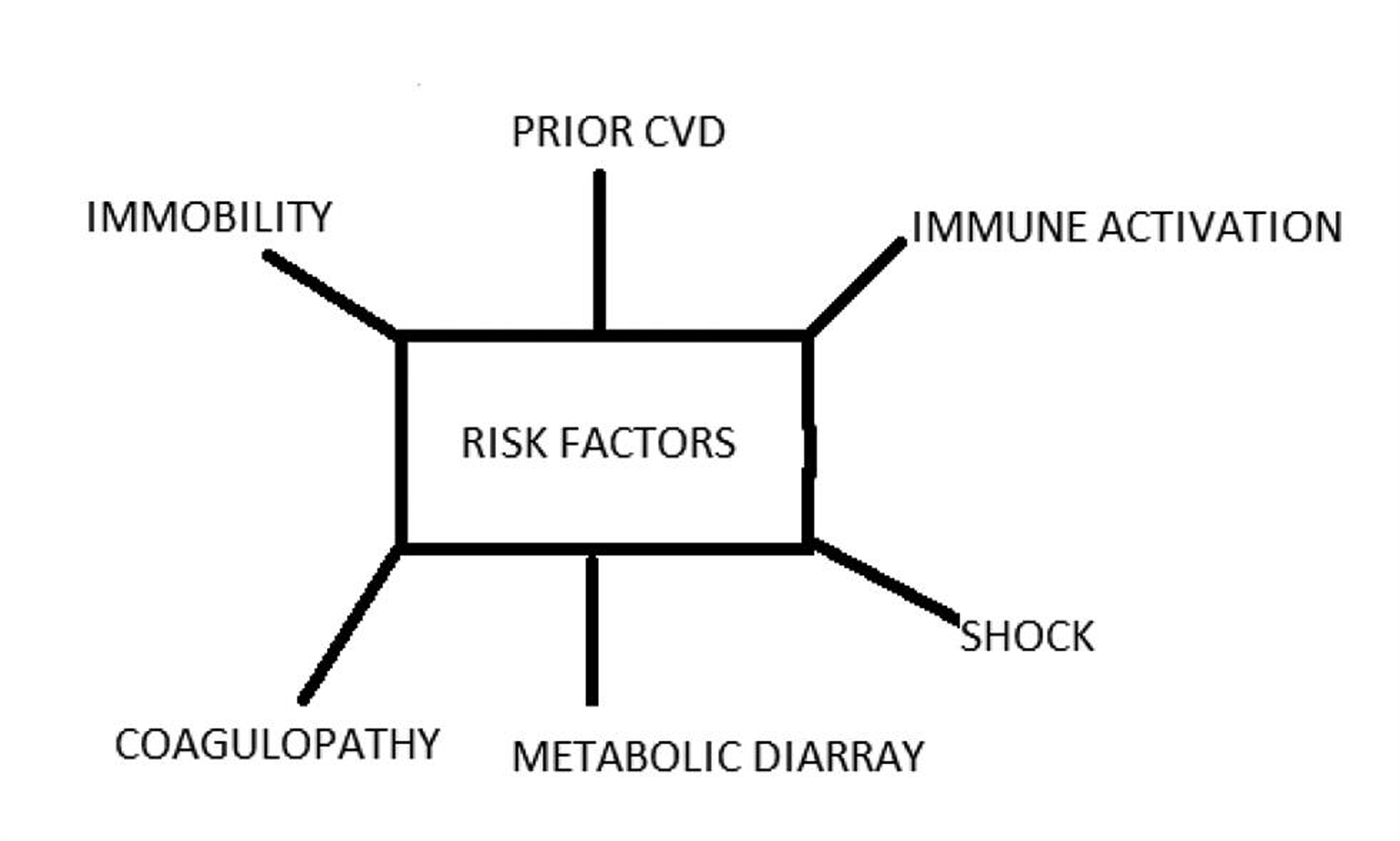
-
Fig. 2 Changes caused by COVID-19, which affect the cardiovascular system.
Fig. 2 Changes caused by COVID-19, which affect the cardiovascular system.
Besides, COVID-19 patients with CVD may have an increase in the severity of underlying heart disease.
-
Heart failure is exaggerated in dilated or ischemic cardiomyopathy patients.
-
Stent thrombosis due to COVID-19 was reported.
-
Arrhythmias may be precipitated in ischemic heart disease or cardiomyopathy patients. The mechanisms are mentioned in Fig. 2.
-
Pulmonary artery hypertension severity may increase in both primary and secondary pulmonary hypertensive patients due to venous thromboembolism on account of COVID-19.
-
May precipitate acute coronary syndrome in stable coronary artery disease. Atherosclerotic plaque rupture may occur in susceptible patients due to the profound inflammatory response and hemodynamic changes associated with severe disease, as demonstrated by Kwong et al in influenza infection26
Effect of CVD on COVID-19
There is a separate review in the same issue mentioning the underlying CVD predisposing to COVID-19. Hypertension, diabetes mellitus and underlying ischemic heart disease are predisposing risk factors for the development of the COVID-19.
Considerations for CV Society
Even though there are definitive guidelines and recommendations for CVD management, they require modification during the COVID-19 era, as all previous recommendations cannot be directly implemented. In Table 3, those modified recommendations by various CV societies are mentioned (Table 3).
|
Society/guideline |
Key recommendations |
|---|---|
|
Abbreviations: ACE, angiotensin-converting enzyme; ARNI, angiotensin receptor–neprilysin inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CVD, cardiovascular disease; PCI, percutaneous coronary intervention. |
|
|
ACC Clinical Guidance27 |
|
|
ESC Council on Hypertension Statement on COVID-1928 |
To continue ACE inhibitor or ARB therapy. |
|
European Society of Hypertension29 |
To continue ACE inhibitor or ARB therapy. In the case of shock, HCWs should continue or discontinue ACE inhibitors and ARB therapy on a case-by-case basis. |
|
Hypertension Canada28 |
Home blood pressure medical regimen to continue |
|
Canadian Cardiovascular Society30 |
Continuation of ACE inhibitor, ARB, and ARNI therapy |
|
Internal Society of Hypertension31 |
Endorse the ESC Hypertension Statement |
|
Are There Any Difference in Gender in these Drug–Drug Reactions |
|---|
|
Conclusions
COVID-19 involves the different components of the CV system, and CVDs themselves constitute a significant risk factor for COVID-19 infections. So, the doctors treating COVID-19 patients should be aware of the drug–drug and drug–disease interaction to safely and effectively manage CVD patients with COVID-19. HCQ and azithromycin have significant drug interactions with other CVD drugs, and the least interaction happens with remdesivir. Other antiviral drugs again require close monitoring, as CVD drug interactions are more. Steroids, which proved to be life-saving in the RECOVERY trial have significant drug–disease interaction in CVD patients. However, whenever either COVID-19 drug or CVD drug is essential to use, with close monitoring of the interactions, drugs can be given. Probably, artificial intelligence (AI) applications in detecting these interactions may be useful in the future than conducting multiple trials to know them.
Conflicts of Interest
None declared.
Reference
- Cardiac Manifestations of Coronavirus (COVID-19) Treasure Island (FL): StatPearls Publishing; 2020.
- [Google Scholar]
- Bergman SJ, Treatment of Coronavirus Disease 2019 (COVID-19): Investigational Drugs and Other Therapies 2020. Available at: https://emedicine.medscape.com/article/2500116-overview
- Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [PubMed:16115318]
- [CrossRef] [Google Scholar]
- Wishart DS, et.al. DrugBank: Hydroxychloroquine, Accession Number DB01611. Available at: https://www.drugbank.ca/drugs/DB01611
- Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(03):269-271.
- [Google Scholar]
- Health RM, Ed. BBC NEWS HEALTH, Coronavirus: What is dexamethasone, and how does it work?; 2020. Available at: HYPERLINK “https://doi.org/10.1038/s41422-020-0282-0%204%20feb,2020” https://doi.org/10.1038/s41422-020-0282-0. Accessed February 4, 2020
- Azithromycin for COVID-19: More Than Just an Antimicrobial? Clin Drug Investig. 2020;40(08):683-686.
- [Google Scholar]
- Tocilizumab in the Treatment of Coronavirus Induced Disease (COVID-19) (CORON-ACT), ClinicalTrials.gov Identifier: NCT04335071
- Faith mokobi, Favipiravir- properties, uses, mechanism, side effects, COVID-19, Jun 25, 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04335071
- COVID-19 Drug Interactions. Available at https://www.covid19-druginteractions.org/checker. Accessed August 19, 2020
- Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 2020 (e-pub ahead of print). doi: https://doi.org/10.1016/j.hrthm.2020.05.014
- Mehra MR, Ruschitzka. F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet 2020 (e-pub ahead of print). doi: https://doi.org/10.1016/S0140-6736(20)31324-6
- FDA: Emergency use Authorization for Hydroxychloroquine and Chloroquine Revoked. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. Accessed June 15, 2020
- FDA. Remdesivir by Gilead Sciences: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of Treatment. Available at: https://www.fda.gov/safety/medical-product-safety-information/remdesivir-gilead-sciences-fda-warns-newly-discovered-potential-drug-interaction-may-reduce. Accessed August 19, 2020
- COVID-19 drug interactions by the University of Liverpool. Available at: https://www.covid19-druginteractions.org/
- Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020 (e-pub ahead of print)
- [CrossRef] [Google Scholar]
- Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus disease 2019 (COVID-19) JAMA Cardiol 2020 (e-pub ahead of print)
- [CrossRef] [Google Scholar]
- Interaction Report from www.covid19-druginteractions.org. Available at at: https://www.covid19-druginteractions.org/BytheUniversityofLiverpool
- A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799.
- [Google Scholar]
- Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(01):23-43.
- [Google Scholar]
- A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293-2303.
- [Google Scholar]
- Concomitant use of statins in tocilizumab-treated patients with rheumatoid arthritis: a post hoc analysis. Rheumatol Ther. 2017;4(01):133-149.
- [Google Scholar]
- Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705-1714. (10238)
- [Google Scholar]
- Need of the Hour— COVID-19 for Cardiologists, CC BY-NC-ND 4.0. Indian J Cardiovascular Disease in Women WINCARS. 2020;5(01):4-7.
- [Google Scholar]
- Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(26):2538-2539.
- [Google Scholar]
- American College of Cardiology. COVID-19 Clinical Guidance for the Cardiovascular Care Team. Accessed Mar 10, 2020, Google Scholar. Available at: https://www.acc.org/~/media/665AFA1E710B4B3293138D14BE8D1213.pdf
- Hypertension Canada's Statement on Hypertension, ACE-Inhibitors, and Angiotensin Receptor Blockers, and COVID-19. Accessed Mar 27, 2020, Google Scholar
- European Society of Cardiology. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. Accessed Mar 27, 2020, Google Scholar
- Canadian Cardiovascular Society. COVID-19 and concerns regarding the use of ACEi/ARB/ARNi medications for heart failure or hypertension Accessed Mar 27, 2020, Google Scholar
- International Society of Hypertension. A Statement From the International Society of Hypertension on COVID-19, Accessed Mar 27, 2020, Google Scholar
- Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013;2(01):77-83.
- [Google Scholar]
- A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Ther Adv Endocrinol Metab. 2014;5(04):77-85.
- [Google Scholar]







