Translate this page into:
Spontaneous Coronary Artery Dissection Postpartum and Recurrence during Menopause: Hormonal Involvement in Acute Coronary Syndromes
*Corresponding author: Dennis Johannes van de Watering, Department of Cardiology, Amphia Hospital, Breda, The Netherlands. dennis_vd_watering@live.nl
-
Received: ,
Accepted: ,
How to cite this article: van de Watering DJ, van den Branden B, Spaan JJ, Meuwissen M. Spontaneous coronary artery dissection postpartum and recurrence during menopause: Hormonal involvement in acute coronary syndromes. Indian J Cardiovasc Dis Women 2023;8:211-4.
Abstract
In this case report, we present a case of a 38-year-old woman with no cardiac history and no risk factors who was admitted to the hospital with a spontaneous coronary artery dissection (SCAD) after pregnancy. Twelve years later, she suffered a recurrent SCAD during her menopause. SCAD is a rare occurrence of acute coronary syndromes and recurrence is even more rare. SCAD seems to occur predominantly in females and seems to be related to hormone levels. At first presentation, the patient was 38 years old and 4 weeks postpartum. She presented with chest pain. Electrocardiogram (ECG) showed loss of r-amplitude but no ST-segment deviation. Troponins were elevated and showed significant rise and fall. She was treated with dual anti-platelet strategy and underwent coronary angiography (CAG) which showed a type 1 dissection of the distal circumflex artery. The lesion was treated conservatively. Second presentation was 12 years later, in which she again presented with chest pain this time during menopause. ECG showed marginal changes and the troponins were again elevated. She underwent CAG again which showed a new SCAD (Type 2 B) in the second marginal obtuse artery. The old SCAD lesion was healed. Renal angiography was preformed which showed an renal bead pattern typical for fibromuscular dysplasia. Again, she was treated conservatively. This case is the first case report of a patient suffering from a recurrent SCAD in a different coronary artery, both events probably related to hormonal changes, that is, postpartum and during menopause. This may emphasize careful examination of chest pain in menopause patients with a history of earlier SCAD.
Keywords
Spontaneous Coronary Artery Dissection
Female
Menopause
Acute coronary syndrome
Vascular diseases
Post Partum
INTRODUCTION
Spontaneous coronary artery dissection (SCAD) is a rare, predominately female occurrence of acute coronary syndromes (ACS).[1] We present a case of a woman who suffered from SCAD postpartum and a recurrent SCAD during menopause 12 years later. The literature will be discussed further on.
CASE REPORT
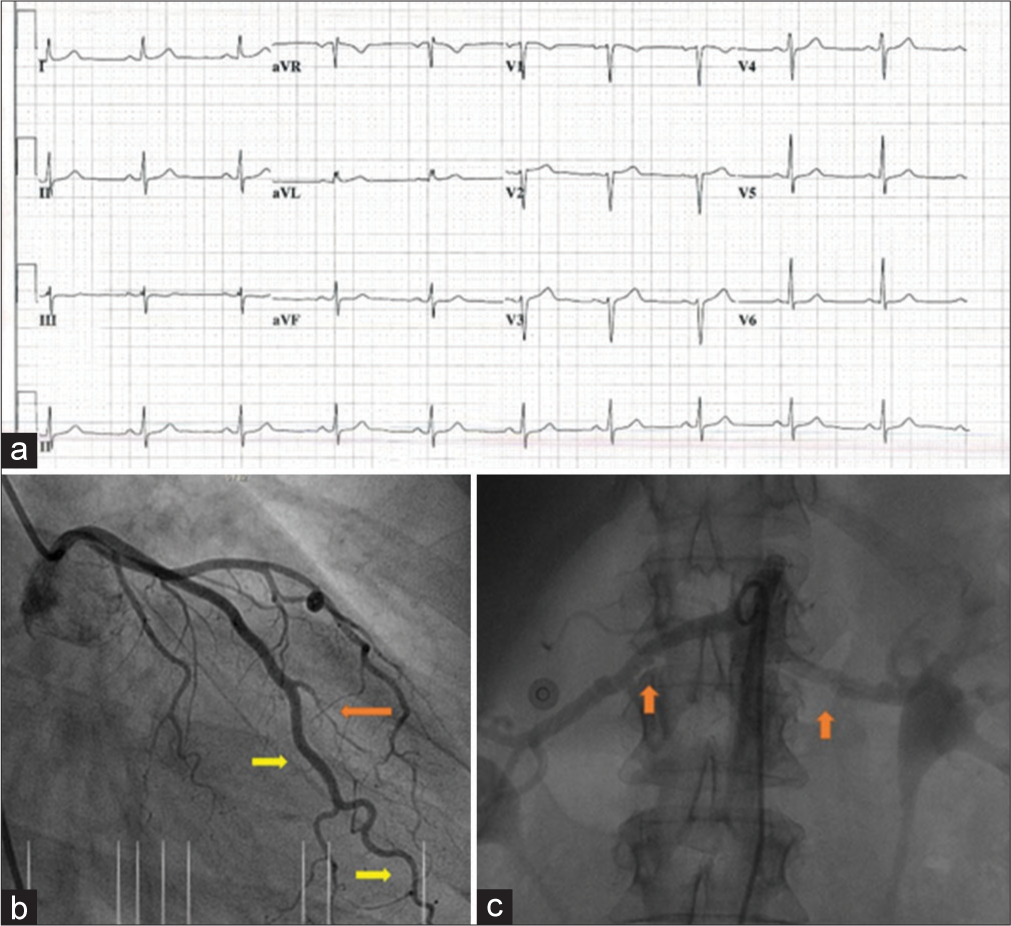
A 38-year-old woman with no cardiac history and no risk factors was admitted to the hospital with transient symptoms of acute chest pain 4 weeks postpartum. Physical examination did not show anything out of the ordinary. Electrocardiogram (ECG) showed loss of R-amplitude in V1-V4 but no ST-segment deviations [Figure 1a]. Blood samples showed elevated c Troponin T of 0.41 (with elevation up to 2.10) and maximum creatinine kinase of 748. According to the ACS protocol, the patient was treated with aspirin, clopidogrel, beta-blockers, and statins. The following day she underwent coronary angiography (CAG), which revealed a long type 1 dissection of the distal circumflex coronary artery [Figure 1b]. The other coronaries were unremarkable. Therefore, the patient was diagnosed with SCAD. Accordingly, the lesion was treated conservatively as there were no chest pain complaints, no ECG changes, and coronary flow was preserved (TIMI 3).[2] Patients pregnancy before the SCAD was uncomplicated without signs of hypertension or pre-eclampsia. The child was 2900 g at birth and partus was at home.

- (a) Electrocardiogram: Sinusrhythm 72/min, intermediate axis, normal conduction, slight loss of R-amplitude in V1-V4, subtle non-significant ST-depression II, and V4-V6. (b) Postpartum coronary angiogram: type 1 and 2B dissection of the mid circumflex and second marginal obtuse coronary artery (right oblique caudal view, yellow arrow. A normal appearance of the first marginal branch was present; orange arrow).
Twelve years later, she returned to the hospital with similar complaints of chest pain. This time patient was in menopause with her last menstruation at the age of 43. She did not use hormonal suppletion. Again, she suffered from an ACS with marginal ECG changes and slightly elevated cardiac biomarkers [Figure 2a] CAG showed characteristics of a new SCAD (Type 2B) with acceptable coronary flow, this time in the second obtuse marginal of the circumflex coronary artery [Figure 2b]. The old SCAD lesion was healed. Again, a conservative approach was followed. Angiography of the renal artery showed a typical “string-of-beads” angiographic pattern of fibromuscular dysplasia (FMD) [Figure 2c]. Echocardiography showed a normal left ventricular function. Two years later, at the out-patient clinic, the patient was symptom free.
- (a) Electrocardiogram: Sinusrhythm 60/min, intermediate axis, normal conduction, ischemic ST depression in inferior leads. (b) Postmenopause coronary angiogram: dissection of the first obtuse marginal coronary artery (orange arrow). A “healed” and normal appearance of the mid circumflex and second marginal obtuse coronary artery was present; yellow arrows. (c) renal arteries with typical angiographic pattern of fibromuscular dysplasia.
DISCUSSION
SCAD is traditionally thought to be a rare occurrence. However, recent studies suggest that the estimated incidence is 0.2–4% of all cases of ACS and this might even be an underestimation.[1,3-8] A Canadian study observed that SCAD accounted for 24% of all the myocardial infarction in woman under 50-years-old.[9] There is a challenge of recognizing SCAD on a CAG. Angiographic appearances and locations vary which can cause the missing of the diagnosis. The different appearances are categorized in three types.
Type 1 shows a classic longitudinal filling defect with a radiolucent intimal flap. Type 2 presents itself with long smooth tubular lesions with no visible dissection plane. Finally, the type 3 which shows multiple focal tubular lesions that resemble atherosclerosis.
The mechanism is that a dissection occurs between the tunica intima and media or between the media and adventitia. The pathophysiology still remains unclear; however, there are some predisposing factors such as the female sex, pregnancy, extreme or unusual physical activity, FMD connective tissue disease, and hormonal therapy.[10-12]
The direct relationship between female sex, pregnancy, and the occurrence of SCAD is unknown; however, it suggests a role of the female sex hormones on the pathophysiology. SCAD can occur at any time during pregnancy and per partum. There are reports of SCAD in woman who are pregnant for 5 weeks up till the third trimester. It also can occur between early to very late postpartum woman (<6 weeks until 24 months thereafter). One role of female hormones on the development of SCAD is that high levels of estrogen and progesterone can cause structural changes in the tunica media.[13] These changes can cause weakening of the coronary artery wall which makes it more prone to dissection. Yet, it is still unknown whether it is caused by fluctuations in hormone levels or the absolute levels.[14]
A Canadian study suggests that pregnancy and peripartum SCAD may present more severe than SCAD outside of pregnancy. These presentations include ST-elevation myocardial infarction, cardiogenic shock, or cardiac arrest, that may lead to maternal death.[15] An increased severity of SCAD during pregnancy was also suggested in a previous literature analysis.[16]
In our case, the patient had a recurrence of SCAD during the menopause. It is unknown whether the menopause may be casual to SCAD because it is a rare occurrence, yet a previous study did show that postmenopausal SCAD shows different clinical and angiographic characteristics.[17] In our patient, hormone levels and specifically estrogen and progesterone were not determined since the patient was already 7 years postmenopause, in which hormone levels are known to be low. It is more likely that non-pregnancy SCAD is associated with predisposing factors. In this case, one of such factors is FMD.
FMD is non-atherosclerotic and non-inflammatory arterial wall disease. It may lead to stenosis, dissection, aneurysms of the coronary arteries but also of the renal, visceral, and cervicocephalic arteries. This disease is documented in 41–86% of patients with SCAD.[18] Therefore, it is important to screen patients with SCAD for FMD. However, there are no therapeutic options besides adequate treatment of hypertension.
The incidence of re-occurrence of SCAD is still in discussion with incidence rates varying between 0.05 and –26.9%.[6,19,20] However, this also seems to depends on definition and on whether extension of the original SCAD or SCAD of the same coronary artery is included.
Our case presents a patient who had a recurrence of SCAD in a different coronary artery at long-term follow-up probably related to hormonal changes during postpartum and menopause which was also associated with FMD.
The optimal management of SCAD remains unclear due to the lack of randomized controlled trials. The management is based on expert opinions. In general, a conservative approach is favorable in this sub group of ACS. Evidence on the value of treating SCAD with dual antiplatelet therapy (DAPT) is lacking.[21] A 2021 Italian study even seemed to show a more than two-fold increased risk of major adverse cardiovascular events in patients receiving DAPT compared to single antiplatelet therapy.[22]
Coronary intervention is reserved for patients with angina, ECG abnormalities, and a large area of risk, for example, in left main coronary dissection or cardiogenic shock. In our patient, an initial conservative approach was followed which showed a spontaneous healing of the first SCAD, which was demonstrated at the follow-up angiogram at the presentation of the second SCAD. Two years later after the second SCAD, at the outpatient clinic, our patient was symptom-free and the echocardiogram showed a normal left ventricular function. A control angiogram was not performed due to the higher risk of iatrogenic dissections in SCAD.[23] CT angiography may be useful for follow-up; however, the resolution is inferior for adequate review nowadays.
CONCLUSION
This is the first report of a patient suffering from a recurrent SCAD in a different coronary artery, both events probably related to hormonal changes, that is, postpartum and during menopause. This may emphasize careful examination of chest pain in menopause patients with a history of earlier SCAD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: Results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250-4.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous coronary artery dissection in the postpartum period. Neth Heart J. 2008;16:412-4.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneuous coronary artery dissection: A Western Denmark heart registry study. Catheter Cardiovasc Interv. 2009;74:710-7.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneuous coronary artery dissection: Novel diagnostic insights from large series of patients. Circ Cardiovasc Interv. 2014;7:638-41.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome: A single-centre Australian experience. Int J Cardiol. 2016;202:336-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic impact of spontaneuous coronary artery dissection in young female patients with acute myocardial infarction: A report from the angina pectorismyocardial infarction multicenter investigators in Japan. Int J Cardiol. 2016;207:341-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5:263-70.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneuous coronary artery dissection: Current state of the science: A scientific statement from the American Heart Association. Circulation. 2018;137:e523-57.
- [CrossRef] [Google Scholar]
- Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814-9.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous coronary artery dissection. Circ Cardiovasc Interv. 2014;7:645-55.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous coronary artery dissection: JACC state-ofthe-art review. J Am Coll Cardiol. 2020;76:961-84.
- [CrossRef] [PubMed] [Google Scholar]
- Physical activity and exercise in patients with spontaneous coronary artery dissection and fibromuscular dysplasia. Eur Heart J. 2021;42:3825-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy-related spontaneous coronary artery dissection. Circulation. 2014;130:1915-20.
- [CrossRef] [PubMed] [Google Scholar]
- The evidence on estrogen, progesterone, and spontaneous coronary artery dissection. JAMA Cardiol. 2019;4:403-4.
- [CrossRef] [PubMed] [Google Scholar]
- A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart. 2016;102:1974-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy and the risk of spontaneous coronary artery dissection: An analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10:e004941.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous coronary artery dissection and menopause. Am J Cardiol. 2021;148:53-9.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous coronary artery dissection: Prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6:44-52.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of single vs. recurrent spontaneous coronary artery dissection. Asian Cardiovasc Thorac Ann. 2018;26:89-93.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent spontaneous coronary artery dissection in the United States. Int J Cardiol. 2020;301:34-7.
- [CrossRef] [PubMed] [Google Scholar]
- Erratum to spontaneous coronary artery dissection: From expert consensus statements to evidence-based medicine. J Thorac Dis. 2019;11:E19.
- [CrossRef] [PubMed] [Google Scholar]
- Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J. 2021;42:3161-71.
- [CrossRef] [PubMed] [Google Scholar]
- Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9:1851-3.
- [CrossRef] [PubMed] [Google Scholar]







