Translate this page into:
Small-vessel Coronary Angioplasty – Past, Present, and Future
*Corresponding author: Sheshidhar Madaka, Department of Cardiology, Nizam's Institute of Medical Sciences, Hyderabad, Telangana, India. sheshidharmadaka@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Madaka S, Cader FA. Small-vessel coronary angioplasty – Past, present, and future. Indian J Cardiovasc Dis Women 2023;8:58-64.
Abstract
Small-vessel disease (SVD) is an important subset of the population with coronary artery disease which may account for up to 30–70% according to different series. The challenges of SVD interventions are first to detect the true small-vessel size along with the true length of the lesion by intravascular imaging, then to decide about the interventional strategies as there is difficulty in delivering the stent to the lesion, dissections, under expansions of the stent during percutaneous coronary intervention with increased incidence of restenosis, and need for revascularization on the long term as well. Special attention is to be paid to this subset of patients of SVD as the treatment strategies need to be improvised than simple balloon dilatation or stenting with drug-eluting stents. At present, drug-coated or eluting balloon emerging as an improvised strategy for treating these patients with intravascular imaging. This article concentrates on the latest updates in SVD treatment.
Keywords
Coronary angioplasty
Drug coating balloon
Small-vessel disease
INTRODUCTION
The definition for small-vessel disease (SVD) has been inconsistent across trials and the published literature. Recently, it has been proposed that a reference vessel diameter of ≤2.5 mm measured by intracoronary imaging should be the cutoff for SVD classification. According to the recent literature, SVD accounts to 30–67% of patients undergoing percutaneous coronary intervention (PCI) according.[1,2] It is more frequent in women, in patients with diabetes mellitus or chronic renal failure[3,4] and in specific coronary anatomic subsets like in bifurcation lesions. In bifurcation lesion, SVD is seen in distal segment and in side branch. The reason for separate discussion of SVD PCI is because of increased risk of restenosis and need of repeated revascularization[5-7] along with the complications during procedure also. Thus, the issue of which small vessels should be intervened on is of paramount importance. In subset of patients with SVD, subtending small myocardial area (i.e., wherein at least 75% of the length of the segment distal to the lesion has a vessel diameter of <2 mm) may not require complete revascularization according to the European Society of Cardiology guidelines. Thus, vessel size is of critical importance in decision-making regarding modality of intervention, and careful evaluation of vessel caliber must be undertaken by appropriate imaging. In this narrative review, we take a look at the recent developments concerning small-vessel disease and the development history with future aspects of small-vessel coronary angioplasty.
UNIQUENESS OF SVD AND INTERVENTION
Patients with SVD constitute separate group among the patients with coronary artery disease (CAD). They are encountered more frequently among elderly diabetics, women, those with peripheral vascular disease and heart failure. The lesions of SVD are usually very complex with type C lesions along with multivessel involvement. The intervention of small vessels was challenging, largely owing to their distal location where stent negotiation and distal delivery are further hampered by tortuosity and calcification. Furthermore, disease in these small vessels is more diffuse requiring longer stents to cover the entire segment. Small-vessel lumen size leaves little space for error in sizing and stent expansion, underscoring the importance of accurate stent sizing by intravascular imaging. This limits the stent option availability (type, length, diameter, and drug) for these vessels. As such, long-term results are even more disappointing with increased adverse cardiac events mainly due to increased need of repeat revascularization, despite satisfactory initial deployment with imaging.[8,9]
ASSESSMENT OF SMALL-VESSEL DIAMETER
First, it is required to confirm that really the angiographically looking a small vessel is indeed small by intracoronary imaging especially after intracoronary nitroglycerine of 200 µg to negate the small size is due to vasoconstriction. Intravascular ultrasound (IVUS) is useful in assessment of luminal diameter, although IVUS might overestimate dimension assessment, and optical coherent tomography might provide the most accurate assessment. In the absence of intracoronary imaging, the vessel size assessment can be done by computer-assisted quantitative coronary angiography.
THERAPEUTIC STRATEGIES IN PATIENTS WITH SMALL-VESSEL CAD
Symptomatic SVD after optimal medical therapy requires revascularization by PCI. As shown in [Figure 1], initially, PCI with plain old balloon angioplasty (POBA), latter with bare-metal stent (BMS), and currently the drug-eluting stent (DES) implantation, with most recent advances in drug-eluting balloons (DEBs), are evolved as treatment strategies for SVD management. There are recommendations for revascularization for large-vessel CAD, but not for SVD separately. As studies with POBA and stents were discussed in previous article published in this journal, in this article, letter developments in management are discussed.

- Evolution of treatment strategies in small-vessel disease.
DRUG-COATED OR ELUTING BALLOONS IN SVD
Drug-coated balloons (DCBs), which function on the strategy of “leaving nothing behind,” have emerged as an alternative to DES for the treatment of small-vessel CAD [Figure 2]. Conceptually, DEBs provide rapid and high-dose delivery of antiproliferative drugs to the vessel wall. This strategy is advantageous over DES as there is no permanent scaffold/stent left over so, acute as well as late complications related to the scaffold/stent can be prevented. In addition, dual antiplatelet therapy (DAPT) for long duration is required with DEB.
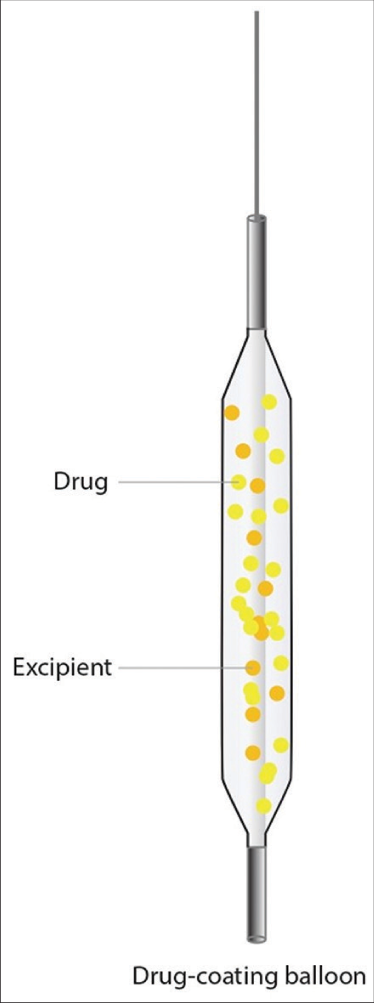
- Drug-eluting balloons model.
Previously used predominantly to treat ISR, there is a growing indication for the use of DCBs in de novo lesions, particularly small-vessel and diffuse vessel disease, which are not appealing for stent implantation. In fact, the use of DCBs has been shown by RCTs and an updated meta-analysis to be similarly efficacious and safer than DES, in anatomic settings such as SVD, wherein DES might result in more adverse outcomes.[10,11] DCBs are also associated with reduced risk of vessel thrombosis as compared with DESs in patients with small-vessel CAD.[10,11]
PACLITAXEL-COATED DCB
Paclitaxel is a highly lipophilic and cytotoxic drug, and selected initially for DCB. The carrier for paclitaxel coating DCB is urea or BHTC or Shellac and coating done with hydrophilic spacer. The mechanism of the drug delivery is by Wander Vaal force of attraction. For proper transport of the drug to the required lesion site if electronic bonds are used, then delivery of the drug becomes difficult due to stronger association, instead if Wander Vaal forces are used to transport as these attractive forces are distance-dependent interaction between atoms or molecules which are weak forces, so drug deliveries very easily and rapidly. The tissue uptake is variable as portioning of drug and drug binding slows transport, leading accumulation of drug.[12]
Paclitaxel-Eluting PTCA-Balloon Catheter to Treat Small Vessel (PEPCAD-I) trial is the first trial to study the use of DCB in SVD. In this trail, SeQuent Please balloon was used to treat SVD lesions. In 30% of patients required bailout BMS stenting and 18% had ISR on 1 year follow up. Following this single-arm study, many single-arm registries reported favorable outcomes with DCBs in SVD.[13-15]
The paclitaxel-coated balloon versus DES during PCI of small coronary vessels (PICCOLETO) trial was first randomized trial with DEB to be stopped, due to unfavorable results of 34% of patients required bailout BMS stenting and proved superiority of DES.[16] However, there were many limitations in this trail. The DEB used was DIOR-I DCB which was the first-generation balloon with low tissue drug delivery, as well the preparation of the lesion before using DEB was not appropriate. More complex cases were included in the study like bifurcation lesions. However, the Balloon Elution and Late Loss Optimization trial in 2012 done with the IN.PACT Falcon DCB showed comparable results as with the Taxus DES. This trail showed good clinical outcomes not only at 6 months, even at 3 years comparable results with DES.[17,18] Meta-analysis of various PCI treatments for SVD by Siontis et al. in 2016 showed most effective results with sirolimuseluting stents followed paclitaxel-eluting stents, then DCBs.[19] Till then, studies available were with first-generation DEBs. Basel Kosten Effektivitats Trial-DCBs versus DESs in Small-Vessel Interventions 2 (BASKET-SMALL 2) trial which included even ACS patients and few observational studies used first-generation DEB with second-generation DES.[20,21]
In RESTORE SVD china, the Restore® DCB (Cardionovum) Paclitaxel-Eluting Balloon which uses SAFEPAX matrix, based on an ammonium salt compound versus RESOLUTE Zotarolimus-Eluting Stent was studied. This is a positive study which demonstrated non-inferiority of DEB over DES. Fifteen months follow-up of 1824 patients with SCD, in an updated meta-analysis, showed DEB better than plain balloon dilatation and comparable results with DES.[22] [Table 1] depicts a summary of RCTs to date comparing DCB versus DES in SVD.
| Study | DCB type | Comparison arm | No. of patients (DCB/comparison arm) |
Follow-up time | Main outcomes |
|---|---|---|---|---|---|
| PICCOLETO I, 2010 | DIOR-I (Eurocor) | Paclitaxel-eluting stent (Taxus Liberte) | 28/29 | 9 months | TLR numerically higher with DCB (32.1 % vs. 10.3 %, P=0.15) MACE higher with DCB (35.7 % vs. 13.8%, P=0.054) trial stopped early |
| Balloon Elution and Late Loss Optimization, 2012 | IN PACT FALCON (Medtronic) | Paclitaxel-eluting stent (Taxus Liberte) | 90/92 | 6 months | Similar binary restenosis (8.9% vs. 14.1%, P=0.25) Similar TLR (4.4% vs. 7.6 %, P=0.37) Similar MACE (7.8% vs. 13.2%, P=0.77) |
| Funatsu et al., 2017 | SeQuent Please (B Braun) | Uncoated balloon angioplasty | 92/41 | 6 months | Lower binary stenosis with DCB (13.3% vs. 42.5%), P<0.01 Similar TLR (3.4% vs. 10.3%, P=0.2) |
| BASKET-SMALL 2, 2018[23] | SeQuent Please (B Braun) | Everolimus-eluting XIENCE stent (Abbott vascular) or Paclitaxel-eluting Taxus Element stent (Boston Scientific) |
382/376 | 12 months | Similar TVR (3.5% vs. 4.5%, P=0.44) Similar MACE (7.5% vs. 7.3%, P=0.92) |
| Restore-SVD China 2018 | Restore (Cardionovum) | Zotarolimus-eluting stent (RESOLUTE, Medtronic) | 116/114 | 9 months | Similar TLF (4.4% vs. 2.6%, P=0.72) |
| PICCOLETO II[24] | Elutax SV (Aachen Resonance, Germany) | Everolimus-eluting XIENCE stent (Abbott vascular) |
118/ 114 | 12 months | In-lesion LLL was significantly lower in DCB versus EES (0.04 vs. 0.17 mm; P=0.001 for non-inferiority; P=0.03 for superiority). Similar 12-month MACE (7.5% vs. 5.6% for DES vs. DCB; P=0.55). |
TLR: Target lesion revascularization, TVR: Target vessel revascularization, DCB: Drug-coated balloon, DES: Drug-eluting stent, MACE: Major adverse cardiac event, LLL: Late lumen loss
Most evidence on DCBs was derived from studies of paclitaxel-coated balloons. In previously discussed studies, the long-term outcomes were not favorable to use only DCB as the primary modality of treatment for CAD, except in side branch lesions after main vessel stenting or in the stent restenosis. More recently, the use of sirolimus-coated balloons was introduced over coming some of the problems of older DEBs.
SIROLIMUS ELUTING DCB
Ideal DCB should have uniform coating, minimal handling damage, low systemic dose, long retention, and low particular matter of the drug. Many of the ideal DCB are met with the sirolimus-eluting DCB (Magic touch) [Figure 3].

- Factors to increase the efficiency of drug-eluting balloons.
Sirolimus has advantages over paclitaxel, that is, it is less lipophilic, cytostatic and has wide therapeutic range. The carrier used to deliver the drug is phospholipid, which is biocompatible and acts as stabilizer, differs from paclitaxel DCB. There is also improvement in the nature of coating (hard coating with encapsulation of the drug with circumferential coating in magic versus easy coating by hydrophilic spacer in sequent please balloon), which facilitated effective drug delivery to the lesion. Mechanism of drug delivery is by Fick’s law of diffusion. Tissue uptake of the drug is also faster from lumen or vasa-vasorum and capillaries due to the smaller submicron particle size of the drug. Nanolute technology (Utilization of Nano Carrier technology for drug delivery by creation of a Core/Shell structure) is predominantly used in this type of DCB which is characterized by submicron carriers, low transit loss of drug, acute drug transfer, and better retention of the drug with targeted drug delivery (due to encapsulation of the drug). By way of mechanism of action, once the DCB is introduced into blood, after balloon inflation, due to change in body pH variation, the drug carrier mimics the body lipids and liberates the sirolimus. About 1.27 µg/ mm2 of sirolimus drug is delivered in 60 s, so ischemic time is also decreased from 3 min to 1 min with this DCB.[25]
In the NANOLUTÈ registry, Sirolimus DEB was done in 156 SVD patients with favorable outcomes. In this registry, MACE was 3.8% and target lesion revascularization/target vessel revascularization was 2.8% at 12 months.[26] The ongoing clinical studies with sirolimus DEB are NANOLUTTE PMS (by Sameer Dani et al.), FASICO and EASTBOURNE (by Bernado Cortese et al.) and Brazil ISR (by Alexander Abizaid et al.), NANOLUTE CIMS (by Keyur Parikh et al.), TRANSFORM I and II (by Antonio Colombo et al.), UKSEB (by Sandeep et al.), JAPAN TRIAL, and SCOPE-ISR and SCOPE-SV (by Leon et al.).
OTHER LIMUS FAMILY DRUG-ELUTING BALLOONS
Biolimus DEB was studied in 212 patients in china which showed positive vascular remodeling was more frequent, and there was a trend toward improved clinical outcomes with this DEB.[27]
DEB WITH MICRONEEDLES
Micro-indentation pressure on DCB is added to increase the efficiency of drug delivery. Compared with conventional DCBs, micro-indentation pressure results from the combination of inflation pressure, coating particle shape, and tissue stiffness. DEB microneedles facilitate longer and better contact with vessel lumen during balloon inflation so that drug delivery is enhanced.[27]
Even though the previous studies were mentioned BA, or stenting with a DES in SVD, at present, DCBs provide an alternative option for these difficult-to-treat lesions with outcomes that are comparable to DES, which is substantiated by the recent meta-analysis by Megaly et al.[26] This is true even in diabetic patients also.[28]
CONCLUSION
Through this review article, we attempt to consolidate the recent advances in small vessel coronary angioplasty, highlighting the promising aspect of the technology along-with the limitations, while highlighting the significance of Drug Coated Balloons. SVD is increasing proportion in the patient with CAD at present and likely to increase due to increasing incidence of diabetes further. Interventions in this of SVD sub group of patients are a great challenge, even with evolving technologies in the stenting as well in pharmacotherapy advancement. Even though acute results are optimal with the present day stent technology, long-term results are not encouraging. Hence, drug-coated balloons evolved as interesting, important, and tenable technology for this SVD patient, due to effective drug delivery without any medium left over, as well as shorter DAPT. Research is ongoing to improve the drug delivery more effectively without compromising the trackability of the balloons like microneedles.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Drug-eluting stent update 2007: Part III: Technique and unapproved/unsettled indications (left main, bifurcations, chronic total occlusions, small vessels and long lesions, saphenous vein grafts, acute myocardial infarctions, and multivessel disease) Circulation. 2007;116:1424-32.
- [CrossRef] [PubMed] [Google Scholar]
- Angiographic and clinical outcome following coronary stenting of small vessels: A comparison with coronary stenting of large vessels. J Am Coll Cardiol. 1998;32:1610-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous coronary intervention for small-vessel coronary disease: Highlight on the everolimuseluting stent. Expert Rev Cardiovasc Ther. 2010;8:1239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153-9.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized trial of paclitaxel-and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27:260-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and angiographic follow-up of small vessel lesions treated with paclitaxel-eluting stents (from the TRUE registry) Am J Cardiol. 2008;102:1002-8.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of restenosis after coronary stent implantation. J Am Coll Cardiol. 1998;31:1291-8.
- [CrossRef] [PubMed] [Google Scholar]
- Periprocedural quantitative coronary angiography after Palmaz-Schatz stent implantation predicts the restenosis rate at six months: Results of a meta-analysis of the BElgian NEtherlands Stent study (BENESTENT) I, BENESTENT II Pilot, BENESTENT II and MUSIC trials. Multicenter ultrasound stent in coronaries. J Am Coll Cardiol. 1999;34:1067-74.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of diabetes on outcome with drug-coated € balloons versus drug-eluting stents: The BASKET-SMALL 2 trial. JACC Cardiovasc Interv. 2021;14:1789-98.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-coated balloons vs drug-eluting stents for the treatment of small coronary artery disease: A meta-analysis of randomized trials. Catheter Cardiovasc Interv. 2021;98:66-75.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99:165-74.
- [CrossRef] [PubMed] [Google Scholar]
- Paclitaxel-coated balloon angioplasty for de novo coronary lesions: A long-term follow-up study. Minerva Cardioangiol. 2016;64:15-22.
- [Google Scholar]
- Treatment of small coronary arteries with a paclitaxel-coated balloon catheter in the PEPCAD I study: Are lesions clinically stable from 12 to 36 months? EuroIntervention. 2013;9:620-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of small vessel disease with the paclitaxel drug-eluting balloon: 6-month angiographic and 1-year clinical outcomes of the Spanish multicenter registry. J Interv Cardiol. 2015;28:430-8.
- [CrossRef] [PubMed] [Google Scholar]
- Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart. 2010;96:1291-6.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: The BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol. 2012;60:2473-80.
- [CrossRef] [PubMed] [Google Scholar]
- 3-year follow-up of the balloon elution and late loss optimization study (BELLO) JACC Cardiovasc Interv. 2015;8:1132-4.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous coronary interventions for the treatment of stenoses in small coronary arteries: A network meta-analysis. JACC Cardiovasc Interv. 2016;9:1324-34.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of very small de novo coronary artery disease with 2.0 mm drug-coated balloons showed 1-year clinical outcome comparable with 2.0 mm drug-eluting stents. J Invasive Cardiol. 2018;30:256-61.
- [Google Scholar]
- Drug-coated balloons: A safe and effective alternative to drug-eluting stents in small vessel coronary artery disease. J Interv Cardiol. 2016;29:454-60.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet. 2018;392:849-56.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-coated balloon versus drug-eluting stent for small-vessel disease: the RESTORE SVD china randomized trial. JACC Cardiovasc Interv. 2018;11:2381-92.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes with drug-coated balloons in small-vessel coronary artery disease. Catheter Cardiovasc Interv. 2019;93:E277-86.
- [CrossRef] [Google Scholar]
- The factors influencing the efficiency of drug-coated balloons. Front Cardiovasc Med. 2022;9:947776.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes with drug-coated balloons vs. drug-eluting stents in small-vessel coronary artery disease. Cardiovasc Revasc Med. 2022;35:76-82.
- [CrossRef] [PubMed] [Google Scholar]
- Biolimus-coated balloon in small-vessel coronary artery disease: The BIO-RISE CHINA study. JACC Cardiovasc Interv. 2022;15:1219-6.
- [CrossRef] [PubMed] [Google Scholar]
- The comparative short-term efficacy and safety of drug-coated balloon vs. drug-eluting stent for treating small-vessel coronary artery lesions in diabetic patients. Front Public Health. 2022;10:1036766.
- [CrossRef] [PubMed] [Google Scholar]







