Translate this page into:
Role of MDCT in Assessment of Long-term Graft Patency in Female Patients
*Corresponding author: Sujata Patnaik, Department of Radiology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India. sujata_patnaik222@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Jayananda AD, Patnaik S, Rao A, Rammurti S. Role of MDCT in assessment of long-term graft patency in female patients. Indian J Cardiovasc Dis Women 2022;7:188-94.
Abstract
Objectives:
The aim of the study was to analyze the utility of CT-coronary angiogram (CT-CAG) in assessment of long-term graft patency compared to conventional invasive coronary angiography (ICA) and highlight any gender-specific issues in female post-coronary artery bypass surgery (CABG) patients.
Materials and Methods:
The medical records and images of 30 patients including four female patients who had CABG in the past and underwent both conventional and CT-CAGs were analyzed retrospectively.
Results:
We studied 30 patients who had CABG in whom both CT-CAG and conventional coronary angiograms were performed. CT-CAG was able to evaluate the grafts better than ICA and was useful even in those which could not be assessed due to technical failure by ICA. There were only four female patients in this series. In first patient, the arterial graft (left internal mammary artery [LIMA]) was patent but distal run off was absent in LAD in CT-CAG images and was reported as poor in conventional coronary angiogram. The second patient had CABG 10 years back. The arterial graft (LIMA) to LAD was patent and there was total block in SVG graft to RCA at proximal anastomotic site. The third patient with hypertension and diabetes also had CABG 10 years back. Both LIMA to LAD and SVG to PDA were patent in her. In last case who had CABG 7 years, two of the three SVG grafts were blocked which were well-demonstrated on CT CAG, including one SVG missed on conventional CAG.
Conclusion:
CT-CAG is a non-invasive and less cumbersome alternative to conventional CAG for the assessment of grafts long after CABG even in women. The data generated by CT-CAG in post-CABG are as good as the invasive CAG and it has distinct advantage of greater acceptability.
Keywords
By-pass grafts
Graft patency
Multi-detector computed tomography
ABSTRACT IMAGE
INTRODUCTION
Long-term clinical outcome of coronary artery bypass surgery (CABG) depends on the patency of conduit vessels grafted to the affected coronary arteries and progression of disease in the native vessels. The assessment of their status after CABG is a critical issue in cardiology practice. Conventional angiography had been the standard method of assessment of graft patency. It is invasive procedure and not totally risk free. During cine-angiography, demonstration of the grafts is operator dependent and at times the hooking of the aortic opening of the SVG grafts is sub-optimal or impossible. Due to recent advances in multi-detector computed tomography (MDCT) with ECG gating, CT-CAG gaining acceptance as an alternative diagnostic technology for the assessment of graft patency in post-CABG patients.[1] Due to smaller size of coronaries and chest wall (breast tissue) attenuation in women, CT-coronary angiogram (CT-CAG) may not be suitable modality for graft patency assessment. This study is aimed at bringing out issues regarding long-term graft patency assessment using CT-CAG and analyze any gender-specific limitations in the female patients.
MATERIAL AND METHODS
Total number of patients who underwent CABG in the past and studied with both conventional invasive coronary angiogram (ICA) and CT-CAG studied for graft patency were 30 of which four were females. CT-CAG was done with 128 slices single-source MDCT SOMATOM Definition system. Patients with heart rates >65 beats/min were given beta-blocker orally 1 hour before the scan. Sublingual nitroglycerine was given to all just before the contrast administration. Patient had to hold breath for 15–24 s. Scan length included supraclavicular fossa to the upper abdomen. Eighty to 100 cc of Iohexol (350 mg/dl) was injected in ante-cubital vein at 5.5 ml/s followed by 40 cc of saline flush. Bolus tracking was used for timing of image acquisition with retrospective ECG gating. Axial, coronal, sagittal, MPL, curved MPR, and volume rendering images were reconstructed and analyzed. Conventional angiograms were obtained within 30 days of the CT-CAG in all cases.
All available conventional ICA and CT-CAG images with patient details and the surgical notes were recorded and a database was created. The evaluability, location, type and status of grafts, degree of stenosis, and presence of occlusion were noted. Any graft not visualized at all compared to surgical notes was also noted. The findings by CT-CAG were compared with those of conventional CAG report.
RESULTS
Among the 30 patients, four were females. Mean age at presentation was 59.2 ± 11.4 years. The mean time-period from surgery to the imaging study was 9.9 ± 1.2 years (3– 25 years). There were total 84 grafts in 30 patients with clear surgical details. Total of 76 grafts were evaluable in both the modalities which are compared further. Most common vessel grafted was LAD and most common arterial graft was left internal mammary artery graft (LIMA) [Table 1]. All the grafts were visualized in CT-CAG, but only 76 out of 84 (90.45%) grafts were visualized in ICA [Table 2]. The eight missed grafts were – three patent arterial, four occluded venous, and one patent venous graft compared to CT-CAG. According to the gold standard ICA, 53 grafts are patent, 19 grafts were occluded, whereas in CTCAG 52 are patent, 22 grafts reported to be occluded. One stenosed arterial graft, one stenosed venous graft, and one patent arterial graft in ICA were interpreted as occluded in CT-CAG. All the grafts reported as patent in ICA were detected and labeled as patent in CT-CAG [Table 3].
| Vessel grafted | Number | Percentage |
|---|---|---|
| Left anterior descending artery | 29 | 34.5 |
| Diagonal | 10 | 12 |
| Left circumflex artery | 02 | 2.3 |
| Obtuse marginal | 18 | 21 |
| Right coronary artery | 13 | 15.8 |
| Posterior descending artery | 09 | 10.9 |
| Ramus | 02 | 2.3 |
| Posterior left ventricular branch | 01 | 1.2 |
| Total number | 84 | 100 |
| Total | Arterial grafts (%) |
Venous grafts (%) |
|
|---|---|---|---|
| As per surgical notes | 84 | 30 (35.7) | 54 (64.3) |
| Evaluable by CT-CAG | 84 | 30 | 54 |
| Evaluable by C-CAG | 76 | 27 | 49 |
CT-CAG: CT-coronary angiogram
| Status | Patent | Stenosed | Occluded | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterial | Venous | All | Arterial | Venous | All | Arterial | Venous | All | ||
| ICA | 23 | 30 | 53 | 2 | 2 | 4 | 2 | 17 | 19 | *1 patent and 1 stenosed arterial graft detected by ICA were interpreted as occluded by CTCAG |
| CT-CAG | 22 | 30 | 52 | 1 | 1 | 2 | *4 | 18 | 22 | |
CT-CAG: CT-coronary angiogram, ICA: Invasive coronary angiography
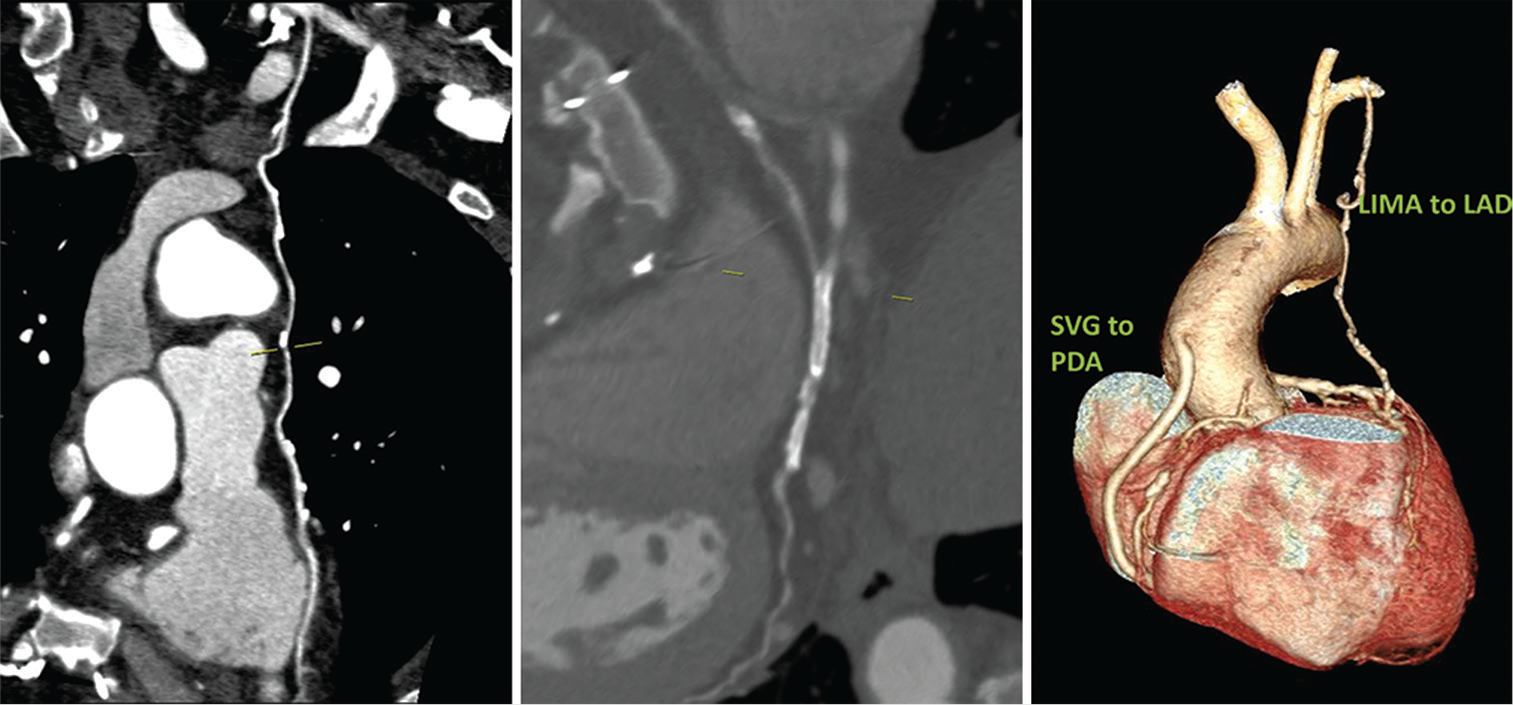
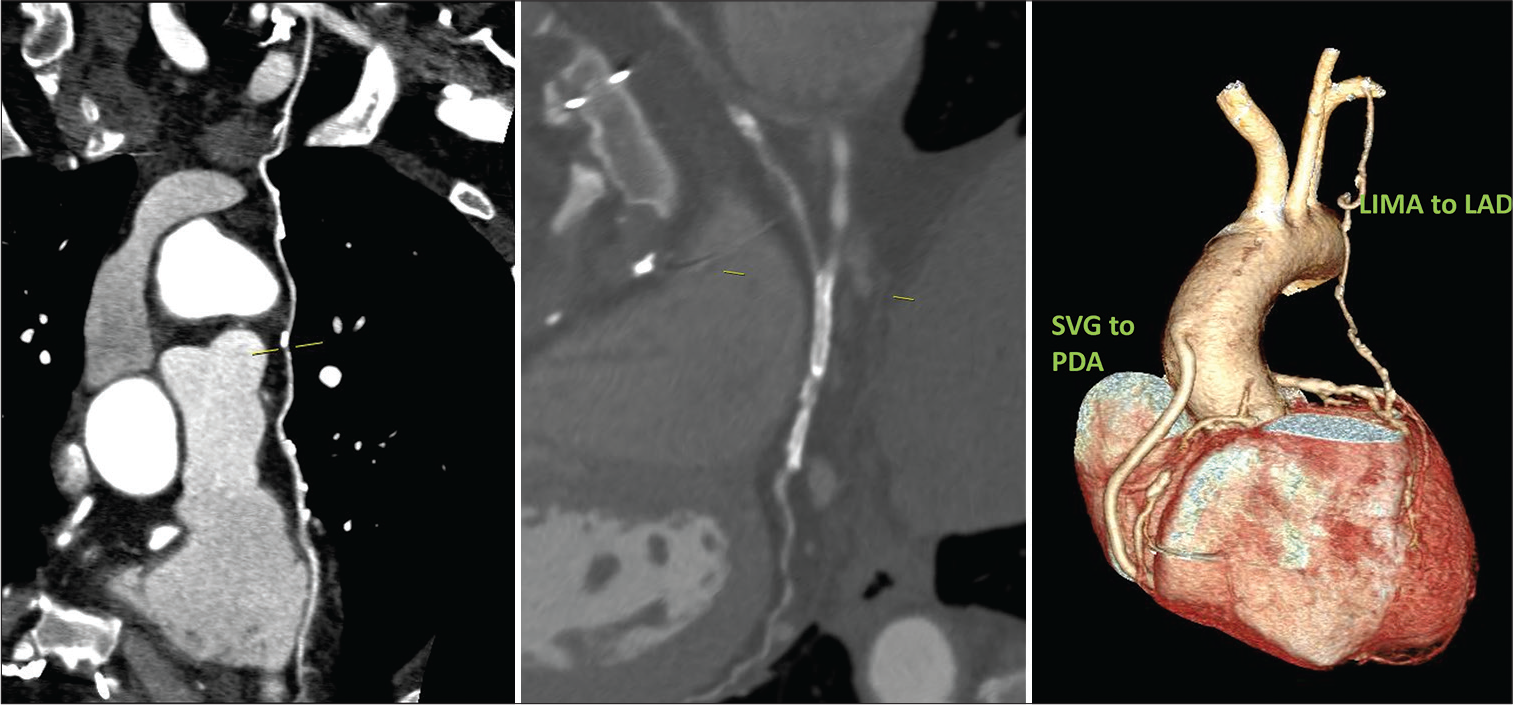
The clinical details and graft patency of four female patients of our series are described below. Case 1: A 50-year-old female had LIMA to LAD arterial graft 8 years ago and presented with shortness of breath for 1 week. She is known hypertensive, diabetic, and hypothyroid. Both CT-CAG and conventional coronary angiograms were performed which confirmed triple vessel disease. The native vessels – RCA, LAD, LCx, and LM were 3.4 mm, 2 mm, 3.1 mm, and 4 mm, respectively, in diameter. The arterial graft (LIMA) is patent but distal run off was absent in LAD in CTCAG images and was reported as poor in conventional coronary angiogram [Figure 1].
- (A) 50/F –CT coronary angiogram (CTCAG) and conventional angiograms reveals- a) Well-opacified LIMA to LAD; b) The same in Volume-rendered reconstruction image; c) Conventional CAG showing LIMA (arrow shows the ostium of LIMA); d) Conventional angiogram showing LIMA (arrow at the anastomotic site). (B) CTCAG showing native RCA with mild disease and diffusely diseased LAD with poor distal run-off.
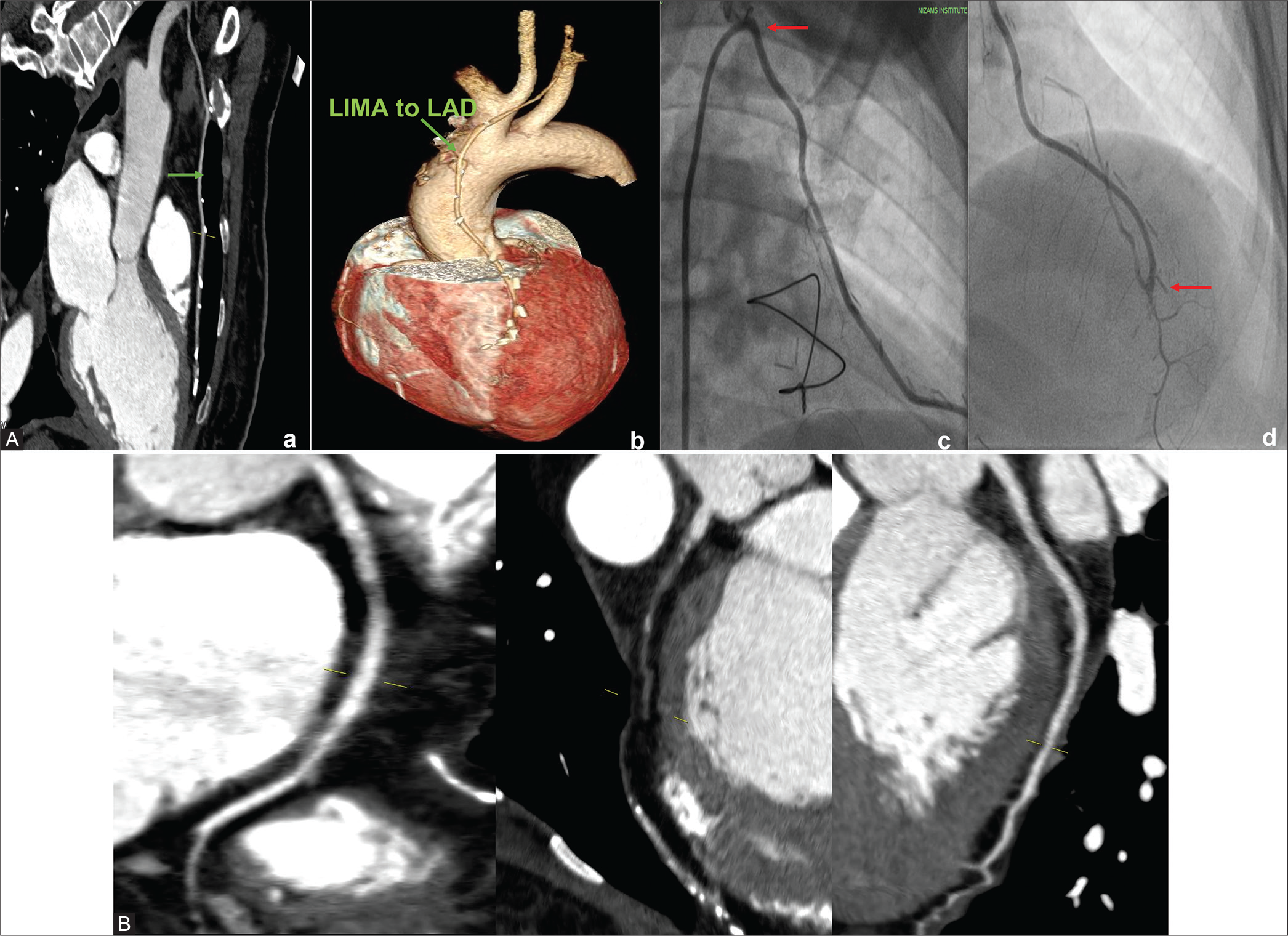
Case 2: A 68-year-old female patient was a known patient with DM and HTN. She had CABG 10 years back with LIMA to LAD and SVG to RCA. Diameter of native vessels – RCA, LAD, LCx, and LM were 3 mm, 1.8 mm, 1.9 mm, and 3.5 mm, respectively. The arterial graft (LIMA) to LAD is patent and there is total block in SVG graft to RCA at proximal anastomotic site [Figure 2].
- (A) CTCAG and conventional CAG –both show well opacification of LIMA to LAD- a) Patent LIMA to LAD on CTCAG; b) Patent LIMA to LAD on volume rendered reconstruction image; c)Conventional angiogram showing LIMA to LAD ( arrow at the ostio-proximal part); d) Nubbin sign (arrow pointing to nipple like structure) on CTCAG showing proximal total occlusion of graft to RCA; e)Anastomotic site (arrow is pointing) LIMA to LAD on conventional CAG; f) Nubbin sign (nipple like structure pointed by arrow) in conventional CAG indicating proximal total graft occlusion. (B) On CTCAG-Native vessels –RCA,LAD and LCx.
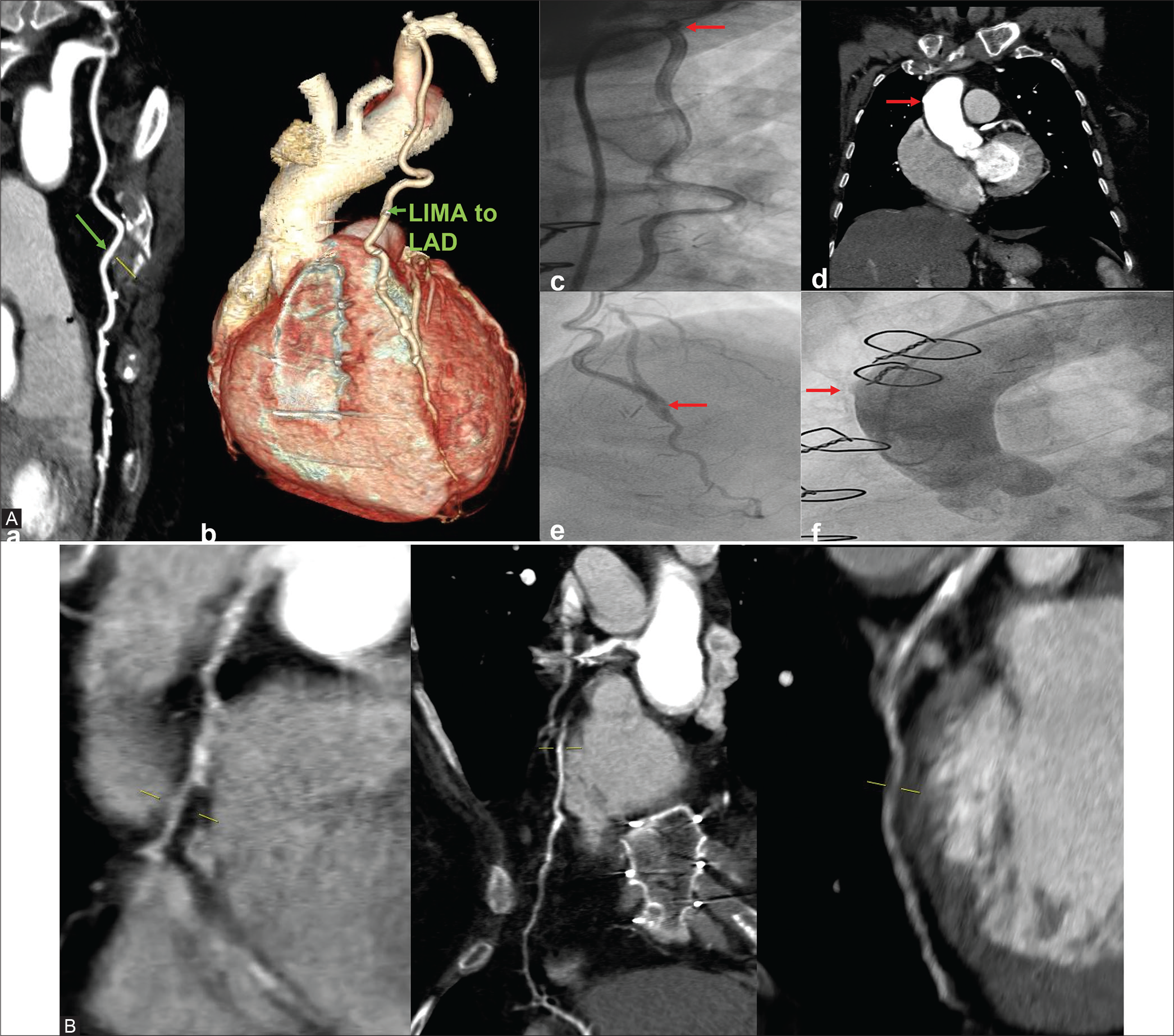
Case 3: A 46-year-old female is a known diabetic and hypertensive. She had CABG 10 years ago following gross restenosis in the stents deployed in RCA and PLVB and new lesions in LAD. As a part of evaluation, she was subjected to CT-CAG and conventional CAG. Both LIMA to LAD and SVG to PDA are patent. The native vessels – RCA, LAD, LCx, and LM showed diameters – 2.5 mm, 2.7 mm, 2.4 mm, and 2.8 mm, respectively. Two stents were seen in RCA and PLVB which showed complete stent occlusion in both [Figure 3].
- 46/F well opacification of bypass grafts with good distal runoff in LIMA to LAD and SVG to PDA as seen in CTCAG (CAG also confirmed the same). Two stents seen in RCA and PLVB with in-stent occlusion in both.
Case 4: A 50-year-old female patient presented with exertional chest discomfort for 1 week. She is a known diabetic and hypertensive and had stenting done to RCA and LCx. Stent in RCA was blocked and stent to LCx was patent. Subsequently due to stent restenosis and new lesion in LAD, CABG was done 7 years ago. Of the saphenous vein graft (SVG) grafts done to OM, LAD, and RCA, only graft SVG to OM is patent and the other two SVG grafts are occluded. Native vessels – RCA, LAD, LCx, and LM are of good caliber measuring 3.2 mm, 3.3 mm, 2.5 mm, and 4.3 mm, respectively, on conventional angiogram. The SVG to RCA was not visualized and the other two were seen with similar findings [Figure 4].
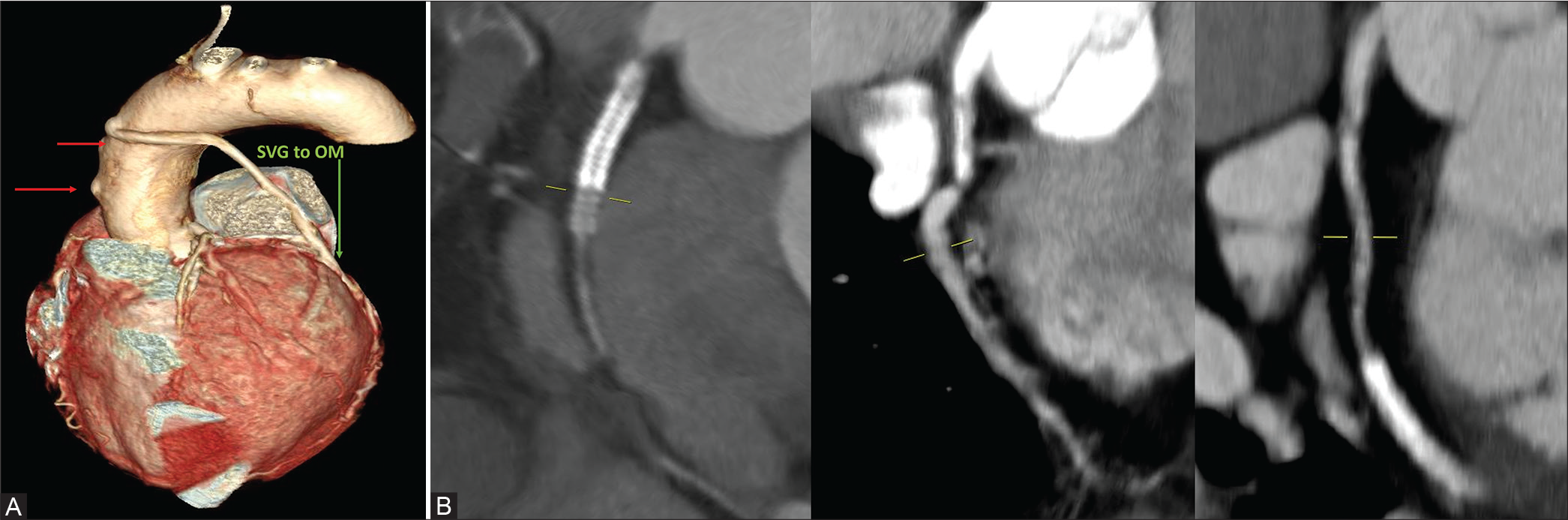
- (A) 50/F- Three SVG grafts to OM, LAD, and RCA. SVG to OM graft is patent and the other two are occluded as indicated by arrows in CTCAG. (B) The occluded stent in RCA and patent stent in LCx.
DISCUSSION
Over years, a range of both arterial and venous grafts are used during CABG to achieve maximum myocardial revascularization. Venous grafts are easily procurable and more readily available compared to arterial grafts. Hence, they are the more commonly used conduits in CABG surgery. The arterial grafts which can be used are LIMA and right internal mammary artery, radial artery, right gastroepiploic artery (RGEA), and inferior epigastric artery (IEA). LIMA is most frequently used as an in situ graft to revascularize the left anterior descending or diagonal artery which supplies the anterior or anterolateral cardiac wall. Venous grafts have an increased tendency to get occluded with time, whereas arterial grafts are more resistant to obstruction as compared to venous grafts. Radial artery graft had higher complications such as graft closure with lower patency rate than that of IMA grafts. It is preferentially anastomosed to the left-sided arteries (OM and LCX). RGEA and IEA are other arterial grafts that are sometimes used in CABG.[2,3] In our series of 30 patients with 84 grafts, the most common vessel grafted was LAD and the most common arterial graft was LIMA [Table 1].
CT-CAG is gaining importance as a choice imaging modality for graft patency evaluation. According to Meyer et al., the sensitivity, specificity, PPV, and NPV for visualization of arterial grafts by CT-CAG were 99%, 97%, 86%, and 98%, respectively, and 99%, 98%, 96%, and 99% for venous grafts in detection of significant bypass graft disease.[4] In patients who underwent CABG, recurrence of symptoms can be due to graft failure or progression of atherosclerosis in the native vessels. The native coronary arteries in patients after bypass grafting are not as easily assessed as are the bypass grafts, and only few studies have addressed the combined reading of coronaries and bypasses. The native arteries are difficult to assess by CT as they often have severe atherosclerosis, including pronounced calcification, and frequently are of small caliber. If the clinical situation requires assessment of the native coronary artery system alone, the value of CTCAG is limited. However, recent scanners with 128 and 256 MDCT have higher temporal and spatial resolution and may thus allow more reliable assessment of the native coronary system in patients with bypass grafts. There are a few studies reporting accuracy of CT-CAG to diagnose stenosis in native ungrafted coronary arteries. Using a 64-MDCT, the reported sensitivity and specificity of CTA in diagnosis of native coronaries were 86–97% and 76–92%, respectively.[5] Despite these results, functional imaging is considered more practical than CT-CAG in evaluation of the status of the native coronary arteries after coronary bypass surgery.
Findings of CTCA are generally compared with ICA, the latter being considered as gold standard. In our study, all 84 grafts as indicated in surgical notes were visualized in CTCA, whereas ICA was able to visualize only 76 grafts (90.45%). In a study conducted by Alberto Trigo Bautista et al., 117 grafts were assessed on the basis of surgical reports, out of which 99 grafts (84.6%) were visualized by ICA and 119(93.2%) were visualized by 16 detector MDCT scanner.[6]
The coronary angiography performed for graft imaging is technically more difficult than for native vessels due to the variation in location of the ostia of the grafts. We also had similar limitations in our experience. All 84 grafts (54 venous and 30 arterial) were visualized by CTCA with 100% evaluability, while eight [three patent arterial, four occluded venous, and one patent venous graft] were missed by ICA [Tables 2 and 3]. None of the grafts had to be excluded from the analysis because of technical limitations such as artifacts on CT-CAG. Various studies conducted by Gorantla et al., Lee et al., Sahiner et al., Khedr et al., and Chaosuwannakit et al. have showed 100% assess ability of grafts in ≥64 slice CT, which is correlating with our study.[7-11]
Causes of graft failure are different in early and late phase. Late-phase venous graft failure is related to exposure to systemic blood pressure leading to neointimal hyperplasia which leads to atherosclerosis beyond 1 year which is the main reason for graft occlusion. Arterial grafts, especially IMA graft, are resistant to atherosclerosis development. Progression of the atherosclerotic disease in the distal runoff vessel more commonly causes late IMA graft failure. Occlusion can be determined by non-opacification of a graft vessel. It is observed that 5 years after CABG, the occlusion rate is 17.6% for saphenous graft and 5.2% for LIMA.[12]
We had patent grafts in 70% of cases (both arterial and venous) similar to the long-term outcome as compared to many other reported studies. Morphological factors such as types of graft, caliber of native coronaries, and degree of stenosis in native vessel that is grafted are key factors for long-term patency.[13] A recent univariate analysis studied – age (>65 years), post-CABG duration >5 years, hypertension, diabetes, dyslipidemia, smoking, diffuse CAD, and LV dysfunction as probable risk factors to predict graft occlusion. Surprisingly, the statistical analysis could not demonstrate any specific risk factor which could significantly be associated with graft patency or occlusion.[14]
There is a common notion that due to smaller size of coronaries and chest wall attenuation due to breast tissue CT-CAG may not be suitable modality for graft patency assessment in women compared to men. It is a surprising observation that there were only four females in our series of 30. There could be multiple socioeconomic factors for such low acceptability of CT-CAG for evaluation of grafts. Our analysis of those four female-patients who had both conventional and CT-CAG brought out few interesting facts. In Case 1, the LIMA graft was patent but the distal run-off was poor after 8 years of CABG. Such late LIMA graft failure may occur from progressive atherosclerotic disease of native grafted vessel distal to anastomosis. Case 2 showed patent LIMA but totally closed SVG. Arterial grafts are known to be patent for longer time than venous grafts.
This is correlating well with several studies which confirmed that the arterial grafts are associated with higher long-term patency as compared to venous grafts.[15] Case 3 is young lady who had CABG at her age of 36 years following stent failure in two lesions. Both LIMA to LAD and SVG to PDA were patent even after 10 years of bypass surgery while SVG grafts failed earlier in Case 4. In this case, conventional CAG could not demonstrate one SVG due to technical difficulty; CT-CAG demonstrated the same with clarity.
CONCLUSION
CT-CAG has many advantages over ICA including lower complication rate, better ostial delineation easy visualization of vessels with anomalous origin, course, and those where ICA failed to demonstrate. CT-CAG, being less cumbersome and cost-effective, should find wider acceptability for female patients. Women who generally shy off from invasive evaluation can greatly benefit from this fast-growing noninvasive technique for graft evaluation.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Audio summary available at
References
- Evaluation of patients after coronary artery bypass surgery: CT angiographic assessment of grafts and coronary arteries. Radiology. 2003;229:749-56.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary artery bypass grafts: Assessment with multidetector CT in the early and late postoperative settings. Radiographics. 2005;25:881-96.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616-26.
- [CrossRef] [PubMed] [Google Scholar]
- Improved non-invasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol. 2007;49:946-50.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of coronary artery bypass grafts and native coronary arteries using 64-slice multidetector computed tomography. Am Heart J. 2007;154:519-26.
- [CrossRef] [PubMed] [Google Scholar]
- Non-invasive assessment of coronary bypass grafts by computed tomography: Comparison with conventional coronary angiography. Rev Esp Cardiol. 2005;58:807-14.
- [CrossRef] [Google Scholar]
- Diagnostic accuracy of 64-slice multidetector computed tomography in evaluation of the post-coronary artery bypass grafts in correlation with invasive coronary angiography. Ind Heart J. 2012;64:254-60.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective versus retrospective ECG-gated 64-detectror coronary CT angiography for evaluation of coronary artery bypass graft patency: Comparison of image quality, radiation dose and diagnostic accuracy. Int Cardiovasc Imaging. 2011;27:657-67.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of dual-source 64-detector computed tomography in evaluation of coronary artery bypass grafts. J Investig Med. 2012;60:1180-5.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of MDCT angiography in assessment of coronary artery bypasses graft. Egypt J Radiol Nuclear Med. 2013;44:183-91.
- [CrossRef] [Google Scholar]
- Diagnostic accuracy of CT angiography in assessment after coronary bypass surgery; evaluation of grafts and native coronary arteries. J Med Assoc Thai. 2014;97:211-9.
- [Google Scholar]
- Contemporary coronary graft patency: 5-year observational data from a randomized trial of conduits. Ann Thorac Surg. 2007;84:795-9.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of veterans Affairs cooperative study. J Am Coll Cardiol. 2004;44:2149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Multislice CT angiography assessment of coronary artery bypass graft (CABG) patients. J Am Sci. 2016;12:92-8.
- [Google Scholar]
- Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1-6.
- [CrossRef] [PubMed] [Google Scholar]







