Translate this page into:
Pulmonary Arterial Hypertension and Pregnancy
Shibba Takkar Chhabra, (DM Cardiology) Department of Cardiology, Dayanand Medical College and Hospital, Unit Hero DMC Heart Institute Ludhiana 141001, Punjab India shibbachhabra@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Pulmonary hypertension in pregnancy has always scared the treating physician and gynecologist due to reported high mortality since ages. The upcoming therapies targeting pulmonary hypertension (calcium channel blockers, nitric oxide, endothelin receptor antagonist, phosphodiesterase type 5 inhibitors) and improvement in hemodynamic monitoring and intensive management in pulmonary arterial hypertension (PAH) specialist centers give a ray of hope to these patients. Termination of pregnancy continues to be a management modality in pregnant patients with PAH. Multidisciplinary approach targeting PAH- and pregnancy-specific therapy in this subset can prove rewarding. Larger multicentric studies in the present era of new pharmacologic agents targeting PAH are required.
Keywords
endothelin receptor antagonist
mean pulmonary arterial pressure
pulmonary arterial hypertension
pulmonary cardiac wedge pressure
pulmonary hypertension
pulmonary vascular resistance
Introduction
Pulmonary hypertension (PH) is a multidisciplinary ailment defined as a sustained elevation in mean pulmonary arterial pressure (mPAP) ≥ 25 mm Hg at right heart catheterization. Pulmonary arterial hypertension (PAH) is a subset of PH described by left ventricular filling pressure of ≤ 15 mm Hg and pulmonary vascular resistance (PVR) > 3 Wood units.1 Intimal fibrosis, increased medial thickening causing pulmonary arterial occlusion, and classic plexiform lesion resulting in high pulmonary vascular pressure characterize PAH. It is commonly seen in women. The mean age of diagnosis is 37 years, and the women-to-men ratio is 1.7:1.2 Idiopathic PAH (IPAH) is encountered occasionally but is rapidly progressing. If left untreated, the median survival age is only 2.8 years,3 and with treatment, the median survival age is 5 to 6 years.
Female PAH patients may become evident for the first time during the course of pregnancy. Pregnancy with PAH has mortality rate as high as 56% in earlier reports in 19th century to 25 to 30% in relatively recent reports.4 Hence pregnancy is not recommended in these patients.
Recent advances show that a multidisciplinary approach to the disease and newer, potent drugs have resulted in a considerable decrease in the mortality rate. The approach may result in a better prognosis and improved quality of life, resulting in healthier outcomes.5
Nonetheless, even with the best new medical therapy, a lot of challenges still have to be overcome. This review article discusses the etiology, pathophysiology, and pertinent management during pregnancy, delivery, and postpartum period. This article is an attempt to look at prevalent clinical practices and suggest the possible amendments in management of PAH based on recent advances.
Hemodynamic Alteration in a Pregnant Woman
Noteworthy hemodynamic alterations occur normally during the course of pregnancy as shown in Fig. 1. These changes develop gradually from fifth week onward and continue up to a few weeks after delivery. Estrogen and progesterone have a vasodilatory effect causing venous distensibility and further decrease in the systemic vascular resistance (SVR). Also, tidal volume is increased despite an elevated diaphragm and normal respiratory rate. This ascent in tidal volume leads to rise in minute volume and alkalosis with a decreased functional capacity.6 Hypercoagulability in pregnancy leads to a surge in production of clotting factors and fibrinogen, decreased production of protein S, and resistance to activated protein C leading to an increased danger of thromboembolic event. The enlarging uterus compresses the inferior vena cava (IVC) resulting in decreased venous return to the right ventricle.7 These factors put stress on the right ventricle leading to an increased possibility of right-sided heart failure in pregnant women with PAH.

-
Fig. 1 Physiologic cardiovascular changes in pregnancy. BP, blood pressure; FAC, factors; PCWP, pulmonary capillary wedge pressure; RBC, red blood cell.
Fig. 1 Physiologic cardiovascular changes in pregnancy. BP, blood pressure; FAC, factors; PCWP, pulmonary capillary wedge pressure; RBC, red blood cell.
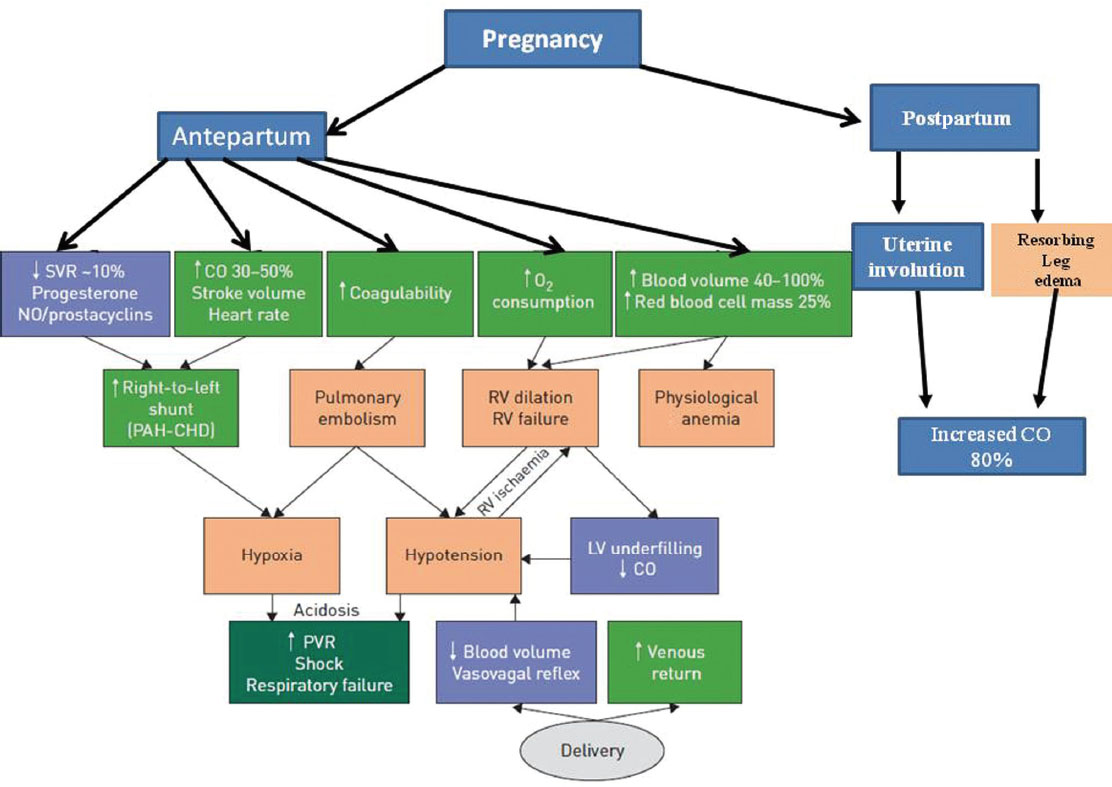
Intra- and peripartum changes in pressure and volume put the patient at a liability of developing right-sided heart failure. Main changes occur in cardiac output (CO) and the blood pressure (BP), especially during labor and delivery. About 500 mL of blood from the uterus gets deflected to maternal circulation with each uterine contraction8 leading to increased CO and systemic BP by approximately 15 to 25%.9 10 Contrary to this, during delivery, the systemic BP might fall due to vasovagal response or increased blood loss that is approximately 500 mL in normal vaginal delivery and 1,000 mL in delivery through cesarean section. CO changes vary across the peripartum period, increasing by 15% in early labor and by 34% during normal vaginal delivery at full cervical dilatation.11 In a cesarean section delivery with spinal anesthesia, it increases by 47% with a 39% decrease in the SVR,6 12 and during postpartum period, it increases up to 80% in lieu of the autotransfusion from the involuting uterus and absorption of pedal edema. These antagonistic factors make the management of labor and delivery immensely complex. In addition, complications during delivery such as hemorrhage, infection, analgesia, and anesthesia can pose immense stress on the woman’s cardiovascular system (Fig. 2).
-
Fig. 2 Pathophysiology in relation to clinical manifestations of pulmonary arterial hypertension (PAH) during pregnancy. CHD, congenital heart disease; CO, cardiac output; LV, left ventricular; PVR, pulmonary vascular resistance; RV, right ventricular; SVR, systemic vascular resistance.
Fig. 2 Pathophysiology in relation to clinical manifestations of pulmonary arterial hypertension (PAH) during pregnancy. CHD, congenital heart disease; CO, cardiac output; LV, left ventricular; PVR, pulmonary vascular resistance; RV, right ventricular; SVR, systemic vascular resistance.
The clinical classification of PH1 (ESC/ERS guidelines 2016) is shown in Fig. 3.

-
Fig. 3 Classification of pulmonary hypertension.
Fig. 3 Classification of pulmonary hypertension.
Chiefly women in the child-bearing age get affected by IPAH and PAH related to connective tissue ailments.13 IPAH is uncommon, rapidly progressing, and fatal if not managed on time. Association with connective tissue disorders is frequent, the highest being with scleroderma, that is, with CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome 10 to 20% and with SLE 10%.14
Pregnancy has a lot of symptoms in common with PAH such as shortness of breath, weakness, fatigue, abdominal discomfort, syncope.15 Sometimes women present with their first symptom of PAH during pregnancy. Hence, high index of suspicion is required on the part of the physician to recognize the symptoms and send the patient for a further evaluation, that is, echocardiography. Assessment of pulmonary pressure by echocardiography is considered an effective screening method.16 However, invasive hemodynamic monitoring, that is, right heart catheterization is required for confirming not only the diagnosis but also looking at how severe the disease pattern is. This helps in determining the prognosis and treatment course. It is the gold standard investigation for PAH with minimum complications as reported by a survey of 7,218 catheterization cases resulting in only 1.1% complications.17
Maternal and Fetal Mortality
The mortality rate in PAH women is considerably high in the studies reported in the past. From 1978 to 1996, a retrospective review study by Weiss18 et al shows that mortality related to primary IPAH is 30%; PH associated with Eisenmenger’s syndrome is 36%; and PH related with other diseases such as connective tissue disorders, liver disease, and thromboembolic phenomenon is 56%.4 With the advances in management techniques and new and potent medication, there seems to be a hope in the decrease in maternal mortality rates. A retrospective study from 1997 to 2015 was undertaken by Ladouceur5 et al on a cohort of 28 pregnant women with PAH stated that mortality rate in PAH women related with congenital heart disease in their cohort is 5%. Silwa19 et al report that mortality due to IPAH is still as high as 43%. Most deaths occurred near delivery or within 1 month post-delivery.20 The figures are prohibitively high so that the best method to manage is discontinuation of pregnancy. The comparison between the studies is limited as they are multicentric with varying levels of quality of care. Severity of the disease also creates a variation in the results.
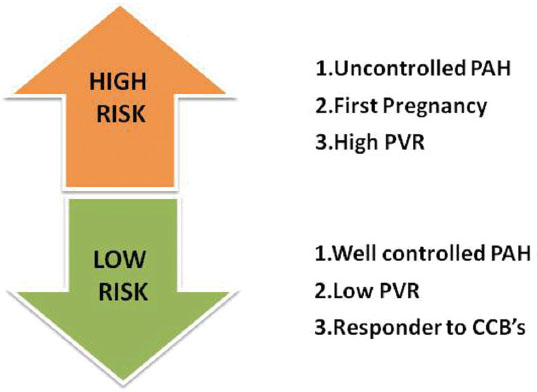
Among the pregnant women with PAH, risk stratification has been done by several authors. Studies outline low-risk pregnancy in PAH women as: PAH effectively managed by PAH-specific therapy, response to calcium channel blockers (CCBs), and low PVR.21 High-risk pregnancies are the ones with uncontrolled PAH, high PVR, and first pregnancy (Fig. 4).21 22 23 A study by Katsuragi24 et al concluded that the level of mPAP prior to and in early stages of pregnancy is a significant indicator of pregnancy outcomes.
-
Fig. 4 Risk stratification of pregnant women with pulmonary arterial hypertension (PAH). CCB, calcium channel blocker; PVR, pulmonary vascular resistance.
Fig. 4 Risk stratification of pregnant women with pulmonary arterial hypertension (PAH). CCB, calcium channel blocker; PVR, pulmonary vascular resistance.
The rate of neonatal complications is high, and the mortality can range from 7% to up to 13% in various reports. The main complications faced include miscarriages 5.6%, fetal death 2%, preterm delivery 21.7%, low birth weight 19%, and neonatal death 0.7%.20 Intrauterine growth restriction (IUGR), maternal distress, and premature delivery are the usual causes of death. Scarce data are available on fetal morbidity.
Counseling Guidelines in Pregnant Women with Pulmonary Arterial Hypertension
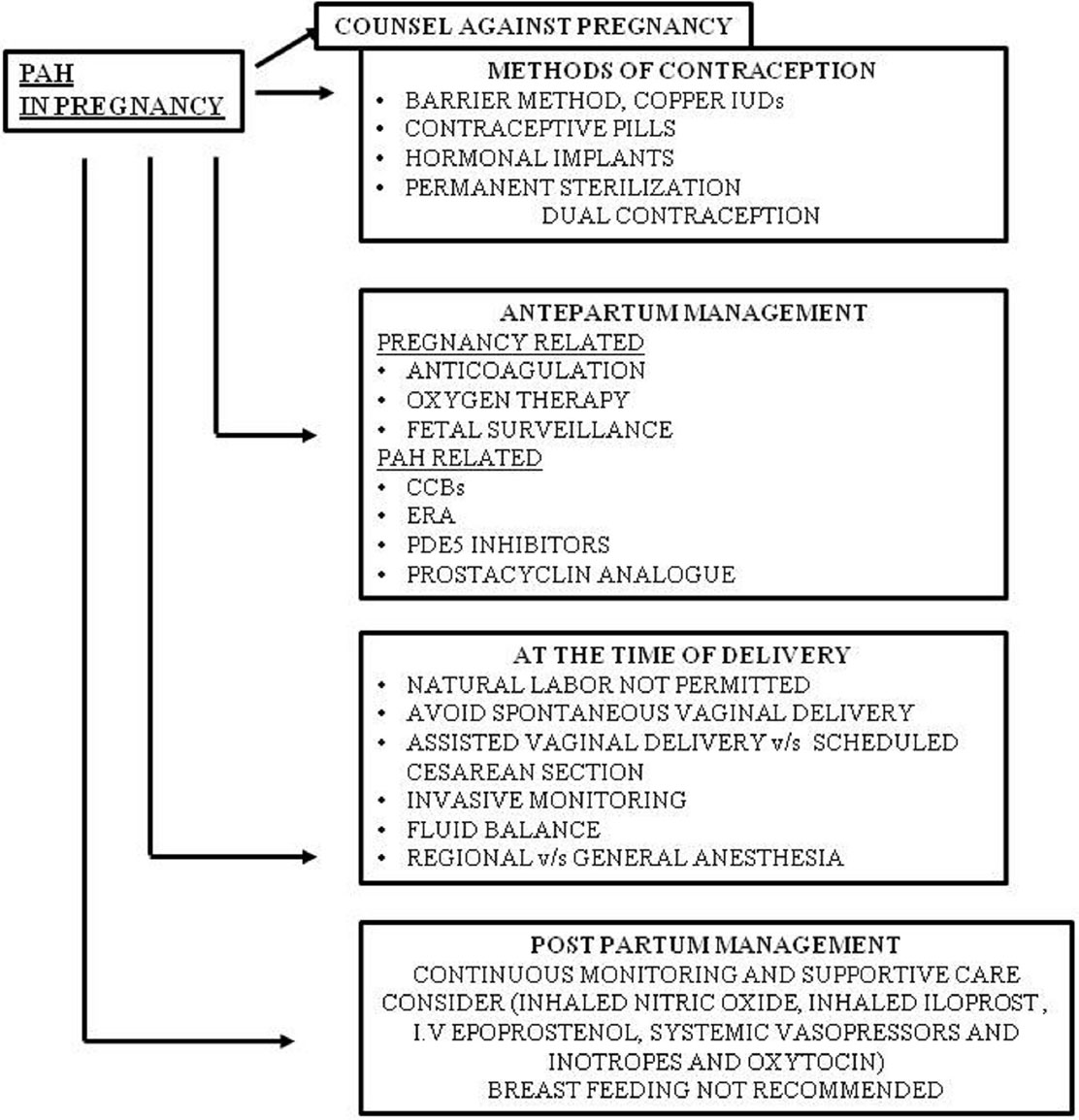
Maternal and fetal risks are high in women of child-bearing age with PAH; therefore, those women are advised against pregnancy.1 9 25 This must be clarified at the time of diagnosis of PAH. They and their families must be counseled at a PH center with expertise in management of pregnancy in PAH.1 7 25 26 These patients must be advised early about the effective contraceptive methods. Barrier methods are safe but unpredictable.1 Intrauterine copper devices (IUCDs) may cause vasovagal reactions during insertions and therefore severe adverse effects.25 26 Hormone-based methods may be used, especially the progesterone-only pills.25 However, the risk of thromboembolic phenomenon might be increased.27 Anticoagulation does not protect entirely against thrombotic events. Permanent methods may not be wanted in many women who may demand temporary contraception. They also bear the risk of complications and anesthesia. Hence dual contraception, that is, using two methods of contraception, is recommended, especially in women on endothelin receptor antagonists (ERAs)—bosentan due to its interactions with progesterone-based methods of contraception.1 7
In vitro fertilization and egg harvesting are not advocated in PAH women due to the possibility of ovarian hyperstimulation and venous thromboembolism (VTE).
If a PAH woman desires pregnancy, genetic screening and counselling must be done,28 especially if it is heritable PAH and/or a mutation in associated gene is discovered. It is important to consider the disease etiology of PAH as it affects the treatment strategies. For example, if PAH is associated with connective tissue disorder being treated with immunosuppressants, pregnancy is contraindicated. Individualization of patients regarding their management during post-pregnancy is important as limited data are available in this sector to generalize the management protocol.
Management Strategies
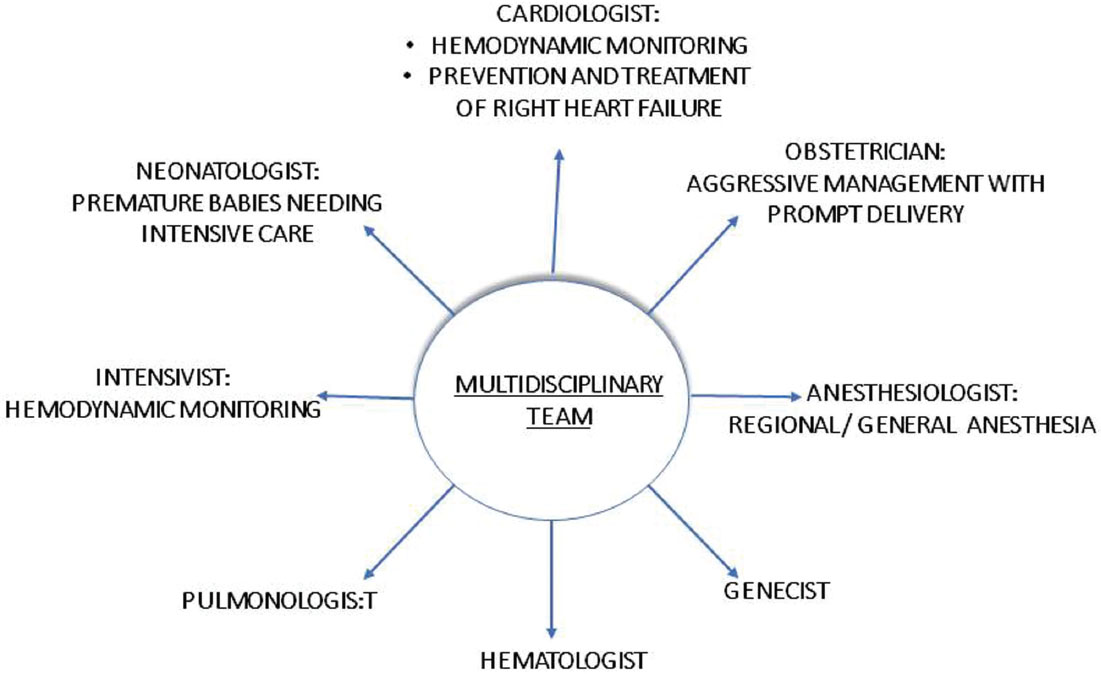
Improvement in survival rates in PAH and pregnancy is a result of advanced management modalities and the utilization of a multidisciplinary approach.15 19 22 29 However, the rates are still prohibitively high so that if pregnancy happens, the woman should be counseled to undergo therapeutic abortion that should ideally take place before 22 weeks of gestation.7 Any delay beyond this has shown to accelerate the risk of mortality. PAH in pregnancy is a complex clinical entity; hence, it should be managed in a PAH specialist center that has expertise in treating PAH in pregnancy and has a collaborative team of an obstetrician, cardiologist, pulmonologist and anesthetist, geneticist, and intensivist (Fig. 5). Women who decide to continue the pregnancy should be kept under close monitoring either by close follow-ups or elective hospitalization. Regular echocardiographic monitoring and fetal evaluation for growth retardation are important alongside the regular clinical evaluation.25 Often, second-trimester hospitalization is pertinent due to increased risk of hemodynamic complications and premature labor.30 31 There is no standard treatment approach to deal with pregnant patients with PAH. Often, the management is tailored according to individual patient’s severity of the ailment and their present condition and should be discussed with the patients priorly.
-
Fig. 5 Multidisciplinary approach toward pulmonary arterial hypertension and pregnancy.
Fig. 5 Multidisciplinary approach toward pulmonary arterial hypertension and pregnancy.
Management of PAH during pregnancy can be dealt with as PAH- and pregnancy-related issues (Fig. 6).
-
Fig. 6 Management of PAH with pregnancy. CCB, calcium channel blocker; ERA, endothelin receptor antagonist; PAH, pulmonary arterial hypertension; PDE5, phosphodiesterase 5.
Fig. 6 Management of PAH with pregnancy. CCB, calcium channel blocker; ERA, endothelin receptor antagonist; PAH, pulmonary arterial hypertension; PDE5, phosphodiesterase 5.
Influence of Pregnancy on Management Strategies
Pregnancy being a hypercoagulable state makes thrombotic arteriopathy a significant marker of PAH and pregnancy that may alter the course and final outcome of the patient.32 Anticoagulation treatment is started in patients suffering from IPAH, heritable PAH, and PAH due to secondary causes. Previous studies show that anticoagulation therapy is given in an average to 52 to 68% women.21 As anticoagulants, vitamin K antagonists are contraindicated as they cause fetal craniofacial malformations during the first trimester, fetal hemorrhage, spontaneous abortion, and malformation of the central nervous system. Warfarin is teratogenic as it crosses the placenta; hence, it is contraindicated. Low-molecular-weight heparin (LMWH) and enoxaparin are the drugs of choice; 1 mg/kg as a subcutaneous injection is given. Heparin is the mainstay of treatment in acute venous thromboembolic event in pregnancy.33 It does not cross the placental barrier; hence, it is safe during pregnancy.
Increased fluid retention and blood volume occur in pregnancy and are also signs of right-sided heart failure; hence, it is important to manage peripheral edema. Patients are suggested to not lie on their backs to avoid pressure on the IVC. Diuretics can be used to manage edema, and torsemide and furosemide are preferred choices. Spironolactone is not given because of its antiandrogenic effects.10 25 A disadvantage of diuretics is that they reduce the blood flow to the placenta; hence, they should be used with caution. Oxygen therapy is given in patients with hypoxia as indicated by blood gas parameters.
For PAH patients insisting to continue the pregnancy, regular monitoring at a center dedicated to treating these patients is advised. At times, elective admission may be considered up to delivery. Besides clinical examination, close monitoring should include regular echocardiograms and assessment of fetal well-being.25 Second-trimester hospitalization may be required due to the possible risk of premature labor and hemodynamic compromize.30 31 Patients should be assessed timely for lung transplantation that may be required in hours of crisis in high-risk patients.
Influence of Pulmonary Arterial Hypertension on Management Strategies
The drugs available for management of PAH and pregnancy include CCBs, ERA, nitric oxide (NO), phosphodiesterase type 5 (PDE5) inhibitors, and prostacyclin analogues. CCBs are the recommended drugs,34 but their use may be limited due to longer half-life and negative inotropic effects.35 Moreover, nifedipine, amlodipine, and diltiazem are pregnancy category C drugs and should be used with caution. Uncomplicated pregnancy is reported in a series of patients on CCBs.21
The current guidelines of European Society of Cardiology and European Respiratory Society advocate that pregnant women with PAH should be continued with PAH-specific treatment1 except for ERAs that being teratogenic should be changed. Therefore, once pregnancy detected, ERA should be stopped and another drug should be started. There is no need to discontinue ERA before pregnancy even in women planning to get pregnant.
Nitric oxide is a potent vasodilator and antiproliferative for pulmonary vasculature. It does so by activating cyclic guanosine monophosphate (cGMP). PDE5 inhibitors inhibit the activation of cGMP by NO and decrease its efficacy. Hence, PDE5 inhibitors inhibit their activity and potentiate the function of NO. Presently used oral drugs are sildenafil and tadalafil.36 They are category B drugs and can be given in pregnancy. Throughout labor NO may be continuous inhaled to decrease the pulmonary arterial pressure (PAP).
Prostacyclin pathway is the most potent route. These drugs can be administered via different routes: Epoprostenol or treprostinil can be given intravenously under supervision (category B pregnancy drugs). Treprostinil can also be given subcutaneously, and iloprost can be administrated by inhalation but is not preferred (category C pregnancy drug).37
If a patient deteriorates on one drug regimen, combination of drugs of different mechanism can be used.38
Delivery Management in Pulmonary Arterial Hypertension
At the time of delivery, PAH women should not go into natural labor ideally, and the delivery should always be planned and under controlled environment. There is a difference in opinion in the literature regarding the appropriate mode of delivery: scheduled cesarean section or assisted normal vaginal delivery.39 Both have their own advantages and disadvantages. In normal vaginal delivery, there is less blood loss (500 mL), less change in blood volume and other hemodynamic factors, less thromboembolic risk, and fewer infections, but the disadvantages include stress and pain period, increased sympathetic activity leading to increased BP, and pulse rate, which puts stress on the right ventricle. Also, labor-induced acidosis, hypoxia, and hypercapnia occur. Regular monitoring of electrocardiogram (ECG), pulse oximetry, central venous pressure, and intra-arterial BP is required; hence, it should be constructed in an intensive care unit (ICU) setting.9 It should be executed preferably in left lateral position to diminish pressure effect on the IVC. Nitrous oxide (N2O) should be avoided as it causes vasoconstriction of the pulmonary vasculature.
On the contrary, cesarean section provides a more regulated environment for delivery. It is done electively under optimal conditions. It avoids lengthy labor but has the disadvantage of the effect of anesthesia and high postsurgical fluid shift.8 19 Cesarean sections are planned around 32 to 36 week of gestation to prevent the woman going into spontaneous labor.
Irrespective of the mode of delivery, PAH should be well controlled. Some centers start intravenous (IV) epoprostenol therapy shortly before delivery, though there are no studies indicating the improvement in results. Even with well-controlled PAH prior to delivery, there have been deterioration and death postpartum; hence, some centers continue IV epoprostenol even after delivery.
Anesthesia
Anesthesia is administrated to prevent pain, hypoxemia, hypercapnia, and acidosis, which increase the PVR and pulmonary pressures.15 It should be given by the trained anesthetist to prevent rise in CO and pulmonary pressure with subsequent uterine contraction. Controversy persists regarding general versus local anesthesia.40
The benefits of regional anesthesia show decreased effect on contractions of the heart and pulmonary resistance, but at the same time, there is a increased bleeding risk as compared with general anesthesia (Table 1).23 41 42
|
Number |
Mode of delivery |
Anesthesia administration |
Maternal mortality |
Fetal mortality |
|
|---|---|---|---|---|---|
|
Abbreviations: CHD, congenital heart disease; CS, cesarean section; IPAH, idiopathic pulmonary arterial hypertension; OPH, other pulmonary hypertension. |
|||||
|
Bédard et al22 |
73 IPAH 29 CHD 29 OPH |
Vaginal 7–30% CS 70–93% |
Regional 28–67% General 29–43% |
17–33% |
10–13% |
|
Kiely et al29 |
10 |
CS |
Regional |
10% |
0 |
|
Jaïs et al21 |
20 |
Vaginal 5% CS 95% |
Regional 80% General 20% |
20% |
10% |
|
Duarte et al23 |
12 |
CS 100% |
Regional 66% General 25% |
16.7% |
0 |
|
Mongale et al42 |
19 |
Vaginal 42% CS 58% |
Regional 50% General 50% |
||
Postnatal Care
Maximum deaths in pregnant women with PAH occur within the first month post-delivery. During delivery there is a surge in the BP both systolic and diastolic due to pressure on the abdominal aorta by the contracting uterus causing autotransfusion and a rise in peripheral vascular resistance. Also, because of relief of caval compression by the gravid uterus, there is temporary increase in venous return, resulting in increased hemodynamic stress and increased incidence of right heart failure. Risk of pulmonary embolism persists post-delivery; hence, continued patient monitoring and supportive management are warranted to avoid right-sided heart failure.43 Postpartum women with PAH, hence, are managed with inhaled NO, inhaled iloprost, IV epoprostenol, systemic vasopressors, and inotropes and oxytocin. However, oxytocin should be used with care as it causes hypotension and reflex tachycardia.
Breast-feeding is to be avoided as pulmonary vasodilators are secreted in the breast milk. Also, prolactin has negative effect on myocardium. Hence close long-term follow-up is recommended.
Babies of PAH mothers are smaller than term babies and should be taken care of in premature care units.
2018 European Society of Cardiology Guidelines for Pregnant Patients with Pulmonary Arterial Hypertension
In accordance with the European Society of Cardiology 2018 guidelines, mortality rate in pregnant women with PAH is approximately 16 to 30%, with the major contributory causes being pulmonary hypertensive crisis, pulmonary thromboembolism, and right-sided heart failure. The fetal and neonatal mortality ranges from 0 to 30%. The guidelines recommend against pregnancy and in favor of contraception and early termination in these patients.44
Patient counseling about the risk and complications of pregnancy and the possibility of inheritance in heritable PAH should be done. If the woman still desires to continue the pregnancy, a regular weekly follow-up is recommended up to third trimester in a PAH specialist hospital. Symptomatic patients are advised bedrest, and supplementary risk factors (like air travel) should be circumvented.
Patients should be risk-stratified as to low versus high risk. Risk factors for maternal mortality include the severity of PAH, late hospital admissions, and administration of general anesthesia. Early combination therapy is favored in pregnant patients with PAH. Patient individualization is recommended. Patients with adequate control on CCBs and vasodilators represent the low-risk subset, and CCBs should be continued in them as should be IV therapies. Bosentan and other ERAs may cause embryopathies; hence, they are contraindicated.
The guidelines also emphasize the role of pregnancy heart team in deciding the optimal time and mode of delivery. Regional anesthesia is recommended along with aggressive peri- and postpartum intensive care.
Gestational Pulmonary Arterial Hypertension
In a few instances, PAH manifest for the first time during pregnancy. This is often difficult to diagnose as symptoms of pregnancy and PAH overlap and requires high index of suspicion so as to start early treatment and avoid complications. Mol et al came across a unique case in which PAH developed only at the time of pregnancies and resolved spontaneously after delivery or termination of pregnancy.45
The etiopathogenesis of gestational PAH could be either an inadequate production of vasodilators causing pulmonary arterial vasodilation or an inadequate response to these mediators. Though the literature cites occasional case reports for gestational PAH, the actual prevalence might be multifold. Hence in symptomatic patients, this possibility should be entertained and echocardiogram reviewed in the postpartum state.
Current data suggest higher complication rates in these patients due to delayed diagnosis and initiation of PAH-related therapies. Depending on the time of diagnosis, termination of pregnancy should be offered to them. Caution is required during interpretation of false-positive echocardiograms secondary to high COs and raised pulmonary pressures during pregnancy.
Indian Studies on Pulmonary Arterial Hypertension
Pulmonary arterial hypertension in pregnancy has several interesting case reports from the Indian subcontinent. Subbaiah et al, in a retrospective cohort of pregnant patients with PAH, reported higher incidence of maternal as well as fetal complications with rising severity of PAH.46 Puri et al reported favorable outcome in a pregnant patient with PAH when managed in a tertiary care center.47 The authors stressed upon adequate management of PAH as well as pregnancy-related issues in these patients with aggressive intensive care.
An interesting case report was published by Hasan et al wherein pulmonary artery pressures significantly abated, following spontaneous development of pulmonary arteriovenous malformation in an IPAH patient.48
Conclusion
Pulmonary arterial hypertension and pregnancy outcomes have been known to be grave with high maternal and fetal mortality. Termination of pregnancy, whenever feasible, continues to be the recommended in this subset of patients. However, with upcoming PAH-specific therapies and development in intensive care modules in PAH specialist centers, pregnancy can be fruitful especially in the low-risk patients. Hence, a multidisciplinary approach with astute, prompt, and aggressive team management is recommended in this subset of patients.
References
- 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(01):67-119.
- [Google Scholar]
- Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012;40(05):1304-1305.
- [Google Scholar]
- Ladouceur M, Benat L, Radojevic J, et al. Pregnancy outcome in patients with PAH associated with congenital heart disease. Heart 2017
- Maternal hemodynamics during cesarean delivery assessed by whole-body impedance cardiography. Acta Obstet Gynecol Scand. 2005;84(04):355-361.
- [Google Scholar]
- Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34(05):681-688.
- [Google Scholar]
- The management of pregnancy and pregnancy-related medical conditions in pulmonary arterial hypertension patients. Int J Clin Pract Suppl. 2011;175(172):6-14.
- [Google Scholar]
- ESC guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(24):3147-3197.
- [Google Scholar]
- Maternal haemodynamic changes during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2011;24(03):242-248.
- [Google Scholar]
- Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43-S54. (1, Suppl)
- [Google Scholar]
- Management of pregnancy in women with pulmonary hypertension secondary to SLE and anti-phospholipid syndrome. Lupus. 2002;11(06):392-398.
- [Google Scholar]
- Correlation of transthoracic echocardiography and right heart catheterization in pregnancy. J Perinat Med. 2007;35(06):497-502.
- [Google Scholar]
- Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48(12):2546-2552.
- [Google Scholar]
- Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998;31(07):1650-1657.
- [Google Scholar]
- Pulmonary hypertension and pregnancy outcome—data from the registry of pregnancy and cardiac disease of the European Society of Cardiology. Eur J Heart Fail. 2016;18(09):1119-1128.
- [Google Scholar]
- Pulmonary arterial hypertension in the setting of pregnancy: a case series and standard treatment approach. Hai. 2012;190(02):155-160.
- [Google Scholar]
- Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012;40(04):881-885.
- [Google Scholar]
- Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009;30(03):256-265.
- [Google Scholar]
- Management of pulmonary arterial hypertension during pregnancy: a retrospective, multicenter experience. Chest. 2013;143(05):1330-1336.
- [Google Scholar]
- Maternal outcome in pregnancy complicated with pulmonary arterial hypertension. Circ J. 2012;76(09):2249-2254.
- [Google Scholar]
- Statement on pregnancy in pulmonary hypertension from the Pulmonary Vascular Research Institute. Pulm Circ. 2015;5(03):435-465.
- [Google Scholar]
- Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32(02):75-81.
- [Google Scholar]
- Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012;345:e4944.
- [Google Scholar]
- Heritable pulmonary arterial hypertension.GeneReviews. Seattle, WA: University of Washington; 2002. In: et al, eds. www.ncbi.nlm.nih. gov/books/NBK1485. Accessed December 17, 2018
- [Google Scholar]
- Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG. 2010;117(05):565-574.
- [Google Scholar]
- Coagulation and anticoagulation in pulmonary arterial hypertension. Isr Med Assoc J. 2009;11(06):376-379.
- [Google Scholar]
- Pulmonary embolism during and after pregnancy. Crit Care Med. 2005;33(10):S294-S300. Suppl)
- [Google Scholar]
- The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327(02):76-81.
- [Google Scholar]
- Arterial pulmonary hypertension in noncardiac intensive care unit. Vasc Health Risk Manag. 2008;4(05):1043-1060.
- [Google Scholar]
- Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361(19):1864-1871.
- [Google Scholar]
- Prostanoid therapy for pulmonary arterial hypertension. Clin Chest Med. 2007;28(01):127-142, ix. ix
- [Google Scholar]
- Combination therapy in pulmonary arterial hypertension. Am J Cardiol. 2013;111(08):16C-20C. Suppl)
- [Google Scholar]
- Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(05):515-521.
- [Google Scholar]
- Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005;102(06):1133-1137, discussion 5A–6A.
- [Google Scholar]
- Pulmonary hypertension and pregnancy: the experience of a tertiary institution over 15 years. Ann Card Anaesth. 2015;18(02):153-160.
- [Google Scholar]
- 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165-3241.
- [Google Scholar]
- Pregnancy outcome in women with pulmonary arterial hypertension: single-center experience from India. Arch Gynecol Obstet. 2013;288(02):305-309.
- [Google Scholar]
- Chhabra S. Primary pulmonary hypertension in pregnancy: a case report. Obst & Gynae Today. 2007;XII(11):507-508.
- [Google Scholar]
- Regression of pulmonary artery hypertension due to development of a pulmonary arteriovenous malformation. Indian Heart J. 2014;66(05):535-538.
- [Google Scholar]







