Translate this page into:
Practice in Medicine: A Rare Presentation of Dilated Cardiomyopathy with Intermittent Complete Heart Block
Daya Shankarlal Vaswani Department of Cardiology, Nizam’s Institute of Medical Sciences (NIMS) Punjagutta, Hyderabad-TS -500 082 India dayavaswani@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Discussant
Dr. Daya Vaswani
Presenter
A 58-year-old female, diagnosed case of hypertension (HTN) for the last 2 years, was on regular treatment with telmisartan and chlorthalidone.
Patient was in her usual state of health until 1 month back when she developed shortness of breath that was exertional in nature initially (New York Heart Association [NYHA] Class II), later progressed to breathlessness even at minimal exertion (NYHA Class III) after 15 days. Patient did not give any history of orthopnea or paroxysmal nocturnal dyspnea (PND) episodes.
Patient also gave history of palpitations for the last 20 days, with sudden onset and offset, not associated with exertion, relieved spontaneously after 20 to 30 minutes. No history of diuresis following palpitations. History of giddiness during episodes of palpitations was present. There was no history of syncope or fall.
There was no history of chest pain, pedal edema, fever, rash, cough, easy fatiguability, weight loss, joint pains, abdominal distension or pain, generalized anasarca, constipation, diarrhea, melaena, tingling numbness of limbs, reduced urine output, burning micturition, or bleeding manifestations.
There was no history of similar episodes in past. Patient is housewife by occupation. Patient consumes mixed diet. There was no history of alcohol intake, smoking, or tobacco chewing. There was no history of use of any other medication other than drugs prescribed for HTN. There was no family history of any cardiac disease or other medical illnesses.
Discussant
Summary of the present illness is, a 58 years old female, diagnosed case of HTN for the last 1 year on regular treatment presented with subacute progression of dyspnea from class II to III in 1 month, palpitations for the last 20 days associated with giddiness during the episode. The clinical presentation of the patient is suggestive of decompensated heart failure and the likely differential diagnoses are as follows:
Dilated Cardiomyopathy
Patients with dilated cardiomyopathy (DCMP) usually present with progressive shortness of breath, but the duration of symptoms is usually longer in these patients, whereas this patient presented with only 1 month history of breathlessness. These patients are also prone to supraventricular and ventricular arrhythmias and hence can present with history of palpitations.1 In fact, onset of new arrhythmia might act as a triggering event for sudden onset of symptoms in previously asymptomatic patient.
Viral myocarditis: Patient presented with sudden onset shortness of breath with a shorter duration of symptoms along with palpitations. Hence, the possibility of viral myocarditis should be strongly considered. However, no inciting event was reported by the patient.
Hypertrophic Cardiomyopathy
Patients with hypertrophic cardiomyopathy (HCM) usually present at an earlier age mostly between third and fourth decade.2 These patients can also present with angina, presyncope, and syncope, or palpitations due to various arrhythmias, most commonly atrial fibrillation (AF) and major contributor for heart failure.3 Late presentation in HCM patients is seen when patients develop burnt-out stage and present as DCMP. A positive family history of cardiomyopathy can be obtained in almost 60% patients.4 This patient presented at 58 years of age with sudden onset symptoms. Possibility of HCM with arrhythmia cannot be ruled out.
Infiltrative Cardiomyopathy
This disorder usually presents after sixth decade with shortness of breath as the presenting complaint and may be associated with palpitations due to strong association with supraventricular arrhythmias. But this cardiomyopathy mostly presents as a component of multisystem disorder.5 Our patient presented earlier and there was no history of involvement of other systems.
Degenerative Aortic Stenosis
Patients with significant aortic stenosis (AS) usually have angina followed by syncope and dyspnea as the initial complaint and AF is the most common arrhythmia associated with AS that might present as palpitations in these patients.6 This patient had no history of angina or syncope.
Degenerative Mitral Stenosis
Mitral stenosis (MS) presents with shortness of breath as the presenting complaint and has AF as a common association at the time of first presentation itself. But dyspnea in this case would be gradually progressive over a longer duration.7 History of PND episodes would strongly favor MS that was not present in this case.
Coronary Artery Disease
Patients with coronary artery disease (CAD) present with angina as the chief complaint but Dyspnea is also considered as an angina equivalent especially if the patient has developed an underlying left ventricular (LV) dysfunction, also predisposing to development of arrhythmias. However, it cannot be considered as an initial differential diagnosis in absence on angina.
Presenter
On examination, patient was alert and oriented. Her body mass index was 32.6 kg/m2. Temperature was 98.4°F. Pulse rate was 46 beats/min, regular; blood pressure was 130/80 mm Hg in right arm supine position. Respiratory rate was 20 cycles per minute and oxygen saturation was 99% while breathing ambient air. There was no pallor, icterus, cyanosis, clubbing, edema feet, or lymphadenopathy. On cardiovascular system examination, jugular venous pressure (JVP) had normal mean column height and cannon “a” waves. Apex beat was localized in left sixth intercostal space along anterior axillary line, hyperdynamic, left ventricular (LV) type. First heart sound was variable in intensity. Second heart sound was normal in intensity and split. No adventitious sounds were appreciated. The lungs were clear. There was no organomegaly on per abdomen examination. Neurological examination was normal.
Discussant
To summarize the examination findings, patient had a pulse rate of 46/min, regular with Cannon “a” waves on JVP and variable intensity of first heart sound pointing toward complete heart block (CHB). There was cardiomegaly with LV dilatation with down and out and hyperdynamic apex beat.
Comprising history and examination findings, the probable diagnosis is as follows:
Infiltrative Cardiomyopathy
Patients with infiltrative cardiomyopathy usually present in heart failure with preserved ejection fraction with concentric hypertrophy in initial stages that can later progress to burnt-out stage and present as DCMP.5 Also, the incidence of CHB and supraventricular tachyarrhythmias is high with this form of cardiomyopathy due to infiltration of conduction system and dilatation of atria. Our patient presented with DCMP with CHB making infiltrative pathology as the most likely underlying mechanism. Sarcoidosis has a predilection for involvement of conduction system but presents more frequently as restrictive cardiomyopathy (RCMP) and amyloidosis usually presents as DCMP with supraventricular tachyarrhythmias.8
Other close differential diagnoses in this case are as follows:
Dilated Cardiomyopathy
DCMP is usually associated with atrial and ventricular tachyarrhythmias. The incidence of degenerative CHB with DCMP has been reported but is not a common finding.9 A holosystolic murmur of mitral regurgitation (MR) may be appreciated at the apex in severe cases of DCMP due to mitral annular dilatation. No murmurs were found in this case.
Degenerative AS
The incidence of degenerative CHB with degenerative AS is around 7.3%.6 But in AS there is concentric LV hypertrophy (LVH) and apex beat is heaving type. Clinically there are no signs of AS.
Hypertrophic Cardiomyopathy
There are case reports of CHB with HCM presenting with uncontrolled HTN and damage to the conduction system but these patients usually present between third to fourth decade of life. HCM presenting late in burnt out stage is usually associated with supraventricular and ventricular tachyarrhythmias.4
Investigations:
-
Chest X-ray (Fig. 1)
-
Electrocardiogram (ECG)

-
Fig. 1 Chest X-ray posteroanterior view.
Fig. 1 Chest X-ray posteroanterior view.

-
Fig. 2 Electrocardiogram on hospital admission.
Fig. 2 Electrocardiogram on hospital admission.
-
Fig. 3 Follow-up electrocardiogram on day 3 of hospital stay.
Fig. 3 Follow-up electrocardiogram on day 3 of hospital stay.
-
24-hour Holter monitoring
-
Two-dimensional echocardiography (2D-ECHO) (Fig. 4)
-
Laboratory investigations (Table 1)
-
Others
-
Fig. 4 PLAX view showing M-mode across left ventricle.
Fig. 4 PLAX view showing M-mode across left ventricle.
|
Biochemical parameter |
Patient range |
|---|---|
|
Abbreviations: aPTT, activated partial thromboplastin time; ESR, erythrocyte sedimentation rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; INR, international normalized ratio; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; NT pro-BNP; N-terminal pro B-type natriuretic peptide; PT, prothrombin time; VLDL, very low-density lipoprotein.
|
|
|
Hemoglobin (gm/dL) |
12.7 |
|
White blood cells (per µL) |
6600 |
|
Platelet count (per µL) |
340000 |
|
Sodium/potassium (mmol/L) |
137/5.1 |
|
Creatinine/urea (mg/dl) |
0.76/50 |
|
Total bilirubin/direct (mg/dl) |
0.9/0.2 |
|
SGOT/SGPT (U/L) |
23/28 |
|
Total protein/albumin (gm/dL) |
7.4/4.2 |
|
PT/INR/APTT (s) |
12/1.02/34 |
|
Total cholesterol/triglycerides (mg/dL) |
138/78 |
|
HDL/LDL/VLDL (mg/dL) |
38/84/16 |
|
hs-CRP (mg/L) |
2 |
|
ESR (mm 1 hour) |
40 |
|
NT pro-BNP (pg/mL) |
1944 |
|
Troponin I |
0.01 |
|
CPK/LDH(U/L) |
51/243 |
|
Serum angiotensin converting enzyme (µL) |
19 |
|
Serum calcium (mg/dL) |
8.6 |
|
Complete urine analysis |
Normal |
|
Serum protein electrophoresis |
Negative |
|
Urine protein electrophoresis |
Negative |
Radiologist
Chest X-ray showing cardiomegaly with LV apex (Fig. 1). Aorta is normal, pulmonary bay is preserved. No pulmonary venous HTN.
Electrophysiologist Opinion
ECG on admission was suggestive of CHB with narrow supra-Hisian escape rate of 46/ min (Fig. 2).
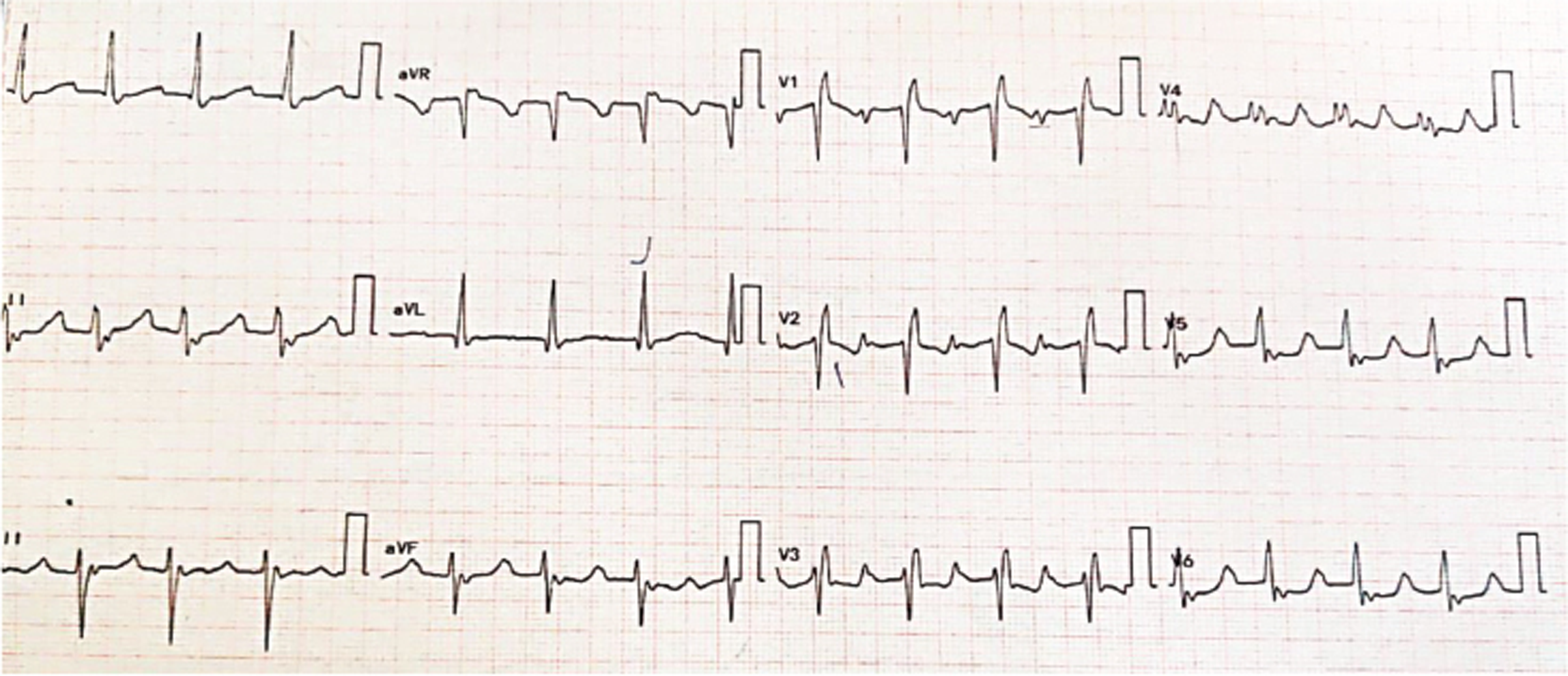
During hospital stay, ECG was suggestive of narrow complex tachycardia with heart rate (HR) of 120/min, regular R-R interval with incomplete right bundle branch block and bizarre P waves most prominent in V1-V3 and occurring midway between RR interval and retrograde P waves in leads II, III, arteriovenous fistula, probably second P wave falling on T wave giving T wave a notched appearance favoring atrial tachycardia (AT) with 2:1 atrioventricular (AV) block (Fig. 3).
ECG on day 3 of hospital stay again showed CHB.
Discussant
So, patient had AV nodal disease with intermittent supraventricular tachyarrhythmia. This type of pattern should raise suspicion for infiltrative cardiomyopathy.
The possible differential diagnoses are as follows:
-
Sarcoidosis
-
Amyloidosis
-
HCM
Sarcoidosis is known to have predilection for the involvement of conduction system presenting as variable degree of AV block. At the same time, these patients are susceptible to atrial and ventricular tachyarrhythmias with supraventricular arrhythmias being more commonly reported mainly AF.10 11
Amyloidosis is frequently associated with supraventricular tachyarrhythmias with AF being the most common arrhythmia. ECG in amyloidosis classically shows low voltage complexes that was not seen in this case.12 Amyloid protein infiltration in the conduction system may result in CHB. Although CHB is seen more commonly with sarcoidosis, the association with amyloidosis is not infrequent.13 14
HCM with degenerative conduction system disease can present with intermittent CHB. HCM patients are more prone to have supraventricular tachyarrhythmias.4 Although AF is most common arrhythmia seen in these patients, other atrial arrhythmias are also known to occur.3 But conduction system disease is usually seen late in the course of disease due to damage to conduction system by altered LV geometry and uncontrolled HTN. Also, LVH should be present in these cases that was not seen in this case.
Presenter
Hence, 24 hours Holter monitoring was done and was suggestive of maximum HR: 144/min; minimum HR: 78/min; 551 ventricular premature complex and 19 supraventricular ectopic and no evidence of ventricular tachycardia)/supraventricular tachycardia/CHB.
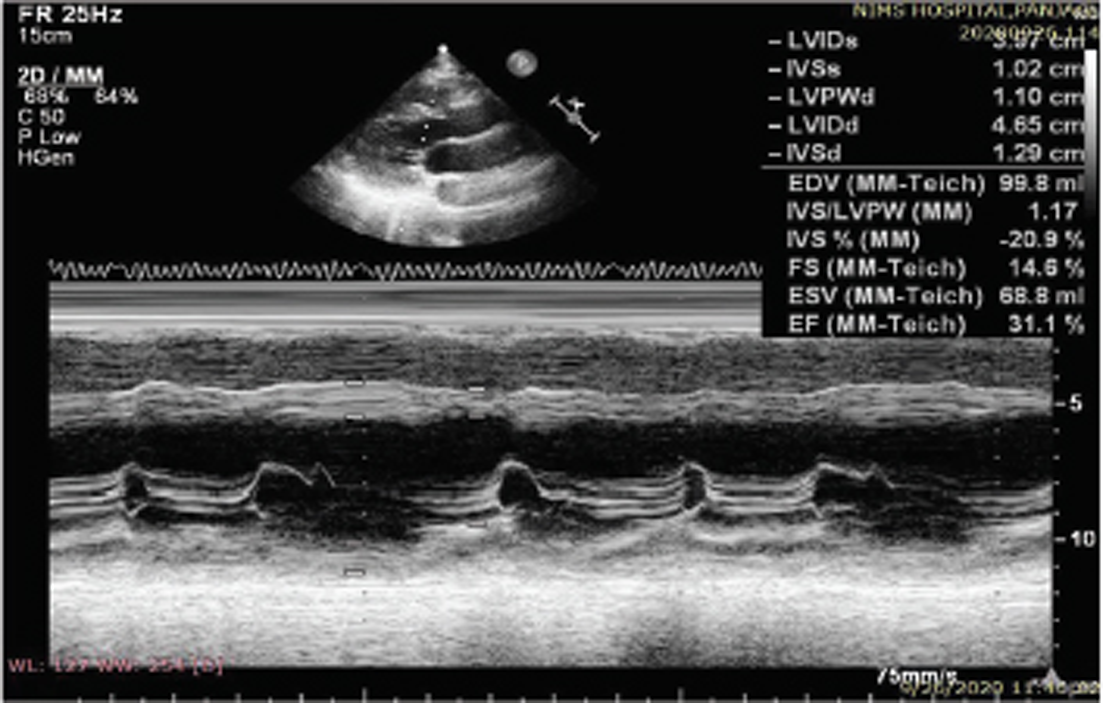
2D-ECHO was done and suggestive of dilated all four chambers, mild concentric LVH (IVS:1.2 cm) with global LV hypokinesia, severe LV dysfunction (ejection fraction: 31%), grade 3 diastolic dysfunction with E wave: 120 cm/s; A: 40 cm/s; E/A: 2:1; DT: 140 ms; E’: 4.8; E/E’ = 25, mild MR/tricuspid regurgitation/pulmonary HTN) with good right ventricle (RV) function (Fig. 4). There was no evidence of sparkling appearance of myocardium with biventricular hypertrophy.
The possible differential diagnoses based on 2D-ECHO findings are as follows:
DCMP
Patient was a diagnosed case of HTN, which might be responsible for mild concentric LVH seen in this case. Patient had dilatation of all four cardiac chambers along with severe LV systolic dysfunction. Patient also had grade 3 diastolic dysfunction with elevated LV end diastolic pressure (LVEDP) suggested by E/E’ ratio of 25. These features favor diagnosis of DCMP.
Late Stage HCM
Patient had concentric LVH with dilated chambers and severe LV systolic and diastolic dysfunction. When HCM presents in late stage, the concentric LVH regresses and patient presents with dilatation of LV and severe LV dysfunction.3
Amyloidosis
Patients with amyloidosis usually have biventricular hypertrophy with thickening of inter atrial septum.15 There is severe concentric LVH with sparkling appearance of the myocardium due to deposition of amyloid protein in the myocardium.16 These patients usually present as severe systolic as well as diastolic dysfunction with elevated E/E’ ratio.17 This patient had mild concentric LVH but no RVH. There was no sparkling appearance of the myocardium, although it is difficult to appreciate with modern echo machine with tissue harmonics. Patient presented with dilated cardiomyopathy with severe LV systolic and diastolic dysfunction with elevated LVEDP. These findings make amyloidosis a strong possibility in this case.
Sarcoidosis
Sarcoidosis usually presents as RCMP with mainly diastolic dysfunction but in case of isolated cardiac sarcoidosis (CS), DCMP is more common than RCMP.18 19
Presenter: Laboratory investigations did not reveal any abnormality (Table 1).
DCMP
Patients with DCMP usually show normal laboratory investigations. N-terminal pro b-type natriuretic peptide (NT pro-BNP) might be raised in these patients.
HCM
These patients do not present with any major laboratory investigation abnormality but can have raised NT pro-BNP in symptomatic patients.4
Sarcoidosis
Sarcoidosis patients usually show anemia with leucopenia, lymphopenia with eosinophilia that was not seen in this patient. Also, hypercalcemia is seen in 63% patients with sarcoidosis.20 This patient had normal serum calcium. Serum angiotensin-converting enzyme (ACE) levels are elevated in 60% patients with systemic sarcoidosis (SS) and 21% patients with CS.21 This patient had normal serum ACE levels. These patients may have raised inflammatory markers. Inflammatory markers were normal in this patient.
Amyloidosis
In suspected cases of cardiac amyloidosis (CA), serum and urine protein electrophoresis with immunofixation (SPEP/UPEP with IFE) as well as kappa and lambda serum free light chains should be tested. Together, these tests are 99% sensitive for a monoclonal plasma cell dyscrasia indicative of light chain type amyloidosis (AL-CA) but are positive only in 20 to 40% of patients with transthyretin type amyloidosis (ATTR-CA).22 23 In our case, both serum and urine electrophoresis with IFE were negative. Inflammatory markers might be raised in these patients.
Other Investigations
-
Coronary angiogram
-
Ultrasonography (USG) abdomen
-
High-resolution computed tomography (HRCT) chest
-
Subcutaneous fat pad biopsy
-
Fluorodeoxyglucose(FDG) positron emission tomography (PET) scan (Figs. 7, 8)
-
Technetium 99m pyrophosphate (Tc 99m PYP) scan (Fig. 9)

-
Fig. 5 Two chamber image showing dilated left ventricle.
Fig. 5 Two chamber image showing dilated left ventricle.

-
Fig. 6 Post contrast 15 minutes delay short axis image showing transmural enhancement of the Interventricular septum (red arrow) and subepicardial enhancement of anterior wall (blue arrow).
Fig. 6 Post contrast 15 minutes delay short axis image showing transmural enhancement of the Interventricular septum (red arrow) and subepicardial enhancement of anterior wall (blue arrow).

-
Fig. 7 Fluorodeoxyglucose positron emission tomography (FDG PET) whole body scan showing increased FDG uptake only in myocardium with normal uptake in other systems.
Fig. 7 Fluorodeoxyglucose positron emission tomography (FDG PET) whole body scan showing increased FDG uptake only in myocardium with normal uptake in other systems.

-
Fig. 8 Fluorodeoxyglucose positron emission tomography (FDG PET) scan showing increased FDG uptake in the entire interventricular septum (red arrow), part of left ventricle lateral wall (blue arrow) and right ventricle (green arrow).
Fig. 8 Fluorodeoxyglucose positron emission tomography (FDG PET) scan showing increased FDG uptake in the entire interventricular septum (red arrow), part of left ventricle lateral wall (blue arrow) and right ventricle (green arrow).
-
Fig. 9 Technetium 99m pyrophosphate scan showing heart/contralateral lung ratio of 1.7 and grade 3 radiotracer uptake in the myocardium compared with ribs.
Fig. 9 Technetium 99m pyrophosphate scan showing heart/contralateral lung ratio of 1.7 and grade 3 radiotracer uptake in the myocardium compared with ribs.
Coronary angiogram was done and showed normal epicardial coronaries.
Discussant
To distinguish the different causes of DCM, cardiac MRI was advised.
Presenter
Cardiac MRI was done to evaluate the cause of systolic and diastolic dysfunction and intermittent CHB with supraventricular arrhythmias.
Radiologist Opinion
There was mild LV dilatation with increased end diastolic diameter (5.7 cm) (Fig. 5) and moderate global LV hypokinesia with severe LV dysfunction (ejection fraction [EF] 34%). Mild global RV hypokinesia was also present. Transmural late gadolinium enhancement (LGE) with basal thinning was noted in basal anteroseptal and inferoseptal and inferior segments and subepicardial LGE was seen in anterior segment and inferior wall of mid ⅓ of LV (Fig. 6). Anterolateral segment showed patchy linear mid myocardial LGE. Basal anteroseptal myocardium on RV side showed >50% LGE thickness. There was predominant basal myocardial segment involvement with thinning favoring CS as the most probable diagnosis.
Discussant
Sarcoidosis: Cardiac MRI in CS typically shows noncoronary nontransmural LGE distribution with predilection for basal and mid ventricular septum with thinning and apical sparing with extensive LGE suggestive of extreme fibrosis.24 This patient had predominantly transmural basal antero- and inferoseptal LGE with basal thinning and subepicardial LGE of inferior mid ⅓, mid anterolateral wall, and >50% LGE on RV wall. Predominant basal involvement was consistent with sarcoidosis but transmural and subepicardial pattern of LGE was not typical of sarcoidosis in this patient.
Amyloidosis: Cardiac MRI in CA typically shows biventricular hypertrophy with atrial septal thickening with diffuse or patchy subendocardial LGE involving atria also.22 This patient had predominantly transmural basal antero- and inferoseptal LGE with basal thinning and subepicardial LGE of inferior mid ⅓, mid anterolateral wall, and >50% LGE on RV wall. The findings were not typical of CA but biventricular hypertrophy with LGE was consistent with CA. Cardiac MRI cannot distinguish between ATTR-CA and AL-CA.
HCM: Cardiac MRI defines the morphology of hypertrophy accurately and distinguishes between various forms of LV hypertrophy. Cine steady-state free precession MRI shows turbulence across left ventricular outflow tract and also nonhypertrophied or thin myocardial regions such as basal crypt in HCM. LGE identifies myocardial replacement fibrosis or scarring that is usually located at the insertion point of LV or RV or the most hypertrophied part of myocardium.25 Thus, cardiac MRI findings in this patient were not consistent with HCM.
DCMP: Cardiac MRI shows LV dilatation with reduced systolic wall thickness and diffuse diastolic wall thinning in advanced cases. Also, the physiologic gradient in systolic wall thickening between LV basal and apical segments disappears in DCMP. LGE shows tissue edema because of inflammatory process in case of myocarditis. There are three patterns of LGE seen in DCMP: (a) no enhancement; (b) subendocardial and transmural involvement consistent with coronary artery distribution and indistinguishable from previous infarction; (c) patchy or longitudinal striae of midwall enhancement clearly different from distribution in CAD patients.26 The LGE pattern seen in this patient was not consistent with any of the patterns described with DCMP.
Thus, the two main differential diagnoses after cardiac MRI were CS and CA. Hence, other investigations are required for the supporting evidence for each of these conditions. Other investigations required are ultrasound abdomen, HRCT of chest, and subcutaneous fat pad or rectal biopsy.
Presenter
USG abdomen was done to look for presence of any organomegaly or lymphadenopathy and was found to be normal. HRCT chest was done to look for the presence of mediastinal lymphadenopathy or lung parenchymal disease but was found to be normal. Subcutaneous fat pad biopsy was also done and was found to be negative.
Discussant
Subcutaneous fat pad biopsy has a sensitivity of up to 80% for detecting AL amyloidosis and is dependent on extent of disease, 45% in ATTRv-CA and only 15% in ATTRwt-CA.22 There was no extracardiac evidence suggestive of sarcoidosis or amyloidosis; in this case, FDG PET whole body scan is recommended.
Nuclear Cardiologist Opinion
FDG PET whole body scan showed cardiomegaly with LV hypertrophy. Intense FDG uptake was noted in entire interventricular septum (IVS) and interrupted FDG uptake noted in lateral wall and RV with faint focal uptake in right atrium (RA). There was no evidence of increased FDG uptake in other systems (Figs. 7, 8).
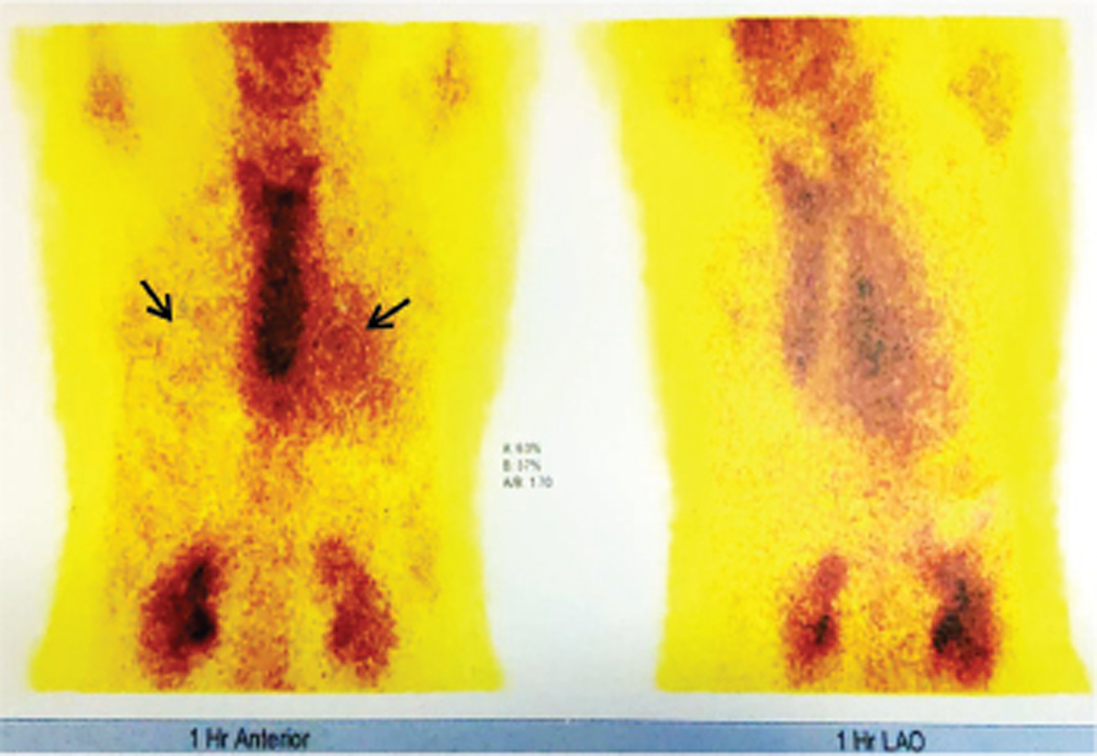
In view of increased FDG uptake predominantly in IVS, which is an important feature of amyloidosis, Tc 99m PYP scan was done to evaluate for presence of amyloidosis. This scan showed heart/contralateral lung (H/CL) ratio of 1.7 and grade 3 radiotracer uptake in myocardium compared with ribs suggesting early transthyretin type amyloidosis (Fig. 9).
Discussant
Sarcoidosis
FDG PET scan in CS can have various patterns like focal uptake, focal on diffuse uptake with the most classic pattern demonstrated in CS being one of “perfusion–metabolism” mismatch, in which areas of F-FDG uptake correspond to areas of reduced or absent perfusion.27 In this case, FDG PET whole body scan showed increased uptake only in myocardial tissue involving entire IVS, with focal involvement of LV lateral wall, RV, and focal uptake in RA. FDG-PET uptake in RA is extremely rare in CS. Also, there was no sign of inflammation elsewhere in the body. Findings were not consistent with classical CS.
Amyloidosis
FDG PET scan in CA shows involvement of both ventricles, predominantly IVS as well as atria and may show increased uptake in other extracardiac tissues.22 In this case, FDG PET whole body scan showed increased uptake in entire IVS with LV lateral wall and RV free wall as well as focal uptake in atria. There was no sign of inflammation elsewhere in the body. These findings were consistent with CA. However, FDG PET scan cannot differentiate between AL-CA and ATTR-CA.
Hence, Tc 99m PYP scan, a radiotracer used for bone scintigraphy, was done. This scan has recently emerged as noninvasive diagnostic test that is 92% sensitive and 95% specific for ATTR-CA in the absence of monoclonal gammopathy.22 One to three hours after injection, single-photon emission computed tomography images are obtained and interpreted with one of two methods. One is the semiquantitative scoring system, which grades radiotracer uptake in the myocardium compared with rib. No myocardial uptake with normal rib uptake is grade 0. Myocardial uptake less than, equal to, or greater than rib uptake is scored as grades 1, 2, or 3, respectively. Grades 2 to 3 are diagnostic of ATTR-CA. The second method, the quantitative scoring system, compares a circular region of interest over the heart (H) to the contralateral lung (CL) field. An H/CL uptake ratio ≥1.5 is also diagnostic of ATTR-CA.23 28 This patient had grade 3 radiotracer uptake with H/CL ratio of 1.7.
Thus, after extensive investigational workup in this patient, a diagnostic dilemma persisted between CS and ATTR-CA. The final step into the diagnostic workup was endomyocardial biopsy (EMB).
Sarcoidosis is an inflammatory granulomatous disease characterized by noncaseating granulomas that can affect any organ but predominantly involves lungs.18 19 Cardiac involvement in sarcoidosis occurs in 20 to 30% patients and is a leading cause of death in these patients.29 30 An estimated 5% of the patients present predominantly with CS without clinical evidence of other system involvement.18 19 Also, the constitutional symptoms such as fever, fatigue, malaise, and weight loss have been observed to occur more often in Indian patients than in patients from the West.31 32 This patient also did not have any other organ involvement and no constitutional symptoms were observed.
Presenter
EMB can establish the diagnosis of CS. But it is known to have low yield in CS due to patchy distribution of noncaseating granulomas and extensive fibrosis.33 Also, in view of its invasive nature and lack of patient’s willingness for the procedure, EMB was not done.
Discussant
Transthyretin type cardiac amyloidosis (ATTR-CA) may be wild type (wild transthyretin type cardiac amyloidosis [ATTRwt-CA]) also called senile type due to deposition of normal transthyretin protein or variant type (ATTRv-CA) caused to deposition of mutant form of transthyretin with ATTRwt-CA (25%) being more common than ATTRv-CA. Usually, ATTRwt-CA is diagnosed at 70 to 75 years of age and has striking male predominance (>90%), while ATTRv-CA presents at a younger age comparatively.34 35 ATTR-CA usually involves peripheral nerves with most common presentation being bilateral carpel tunnel syndrome. This patient was a 58-year-old female with no evidence of involvement of peripheral nervous system.
EMB with Congo red staining is the gold standard for diagnosis of CA and allows for protein typing also.22 But, in this case sufficient information was obtained with noninvasive scan. Hence, biopsy was not done due to its invasive nature and patient also did not give consent for the procedure.
Clinical Impression
This patient had positive Tc 99m PYP scan with negative urine and serum protein electrophoresis thus confirming the diagnosis of ATTR-CA with 92% sensitivity, 95% specificity and 100% positive predictive value. But, patient presented with DCMP with significant AV block and cardiac MRI showed significant basal myocardial involvement. Hence, CS was also kept as an important differential diagnosis.
Clinical Diagnosis
Transthyretin type CA.
Discussion of Management
A staging system for ATTRwt-CA stratifies patients into three stages based only on troponin T (<0.05 ng/mL) and NT pro-BNP (<3000 pg/mL). Stages I, II, and III are defined as having both, one, or neither of the markers below the threshold, with a median survival of 66, 40, and 20 months, respectively.12 Our patient had troponin T of 0.01 ng/mL and NT pro-BNP of 1944 pg/mL. Hence, our patient was classified as being in stage I of disease.
A more recent study of both ATTRwt-CA and ATTRv-CA proposed substituting troponin-T with estimated glomerular filtration rate (eGFR) of 45 mL/min. Median survival was 69, 47, and 24 months in stages I, II, and III, respectively, with longer survival in ATTRwt-CA than in ATTRv-CA.36 Our patient had eGFR OF 82 mL/min/m2. According to this staging system also, patient was in stage I of illness.
Patient was treated on lines of management of heart failure with salt restriction, loop diuretics, mineralocorticoid receptor antagonist, and ACE inhibitors. Beta blockers and calcium channel blockers were not given in view of intermittent CHB. Diflunisal, an FDA-approved nonsteroidal anti-inflammatory drug but also a transthyretin stabilizer,37 was advised to the patient with initial dose of 250 mg BD. Tafamidis, transthyretin stabilizer, is the first-line treatment for ATTR-CA,38 and was advised to the patient but in view of patient’s nonaffordability, diflunisal was started. In view of intermittent CHB and basal thinning on cardiac MRI, patient was advised for cardiac resynchronization therapy.
Final Diagnosis
Transthyretin type CA.
Conflict of Interest
None.
References
- Cardiomyopathy. Diagnosis and management of dilated cardiomyopathy. Heart. 2000;84(01):106-112.
- [Google Scholar]
- New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254.
- [Google Scholar]
- Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517-2524.
- [Google Scholar]
- Hypertrophic cardiomyopathy: gen-etics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(07):749-770.
- [Google Scholar]
- Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101(21):2490-2496.
- [Google Scholar]
- Atrial fibrillation in severe aortic valve stenosis — association with left ventricular left atrial remodeling. IJC Heart & Vessels. 2014;4:102-107.
- [Google Scholar]
- Clinical characteristics, natural history and predictors of disease progression in patients with degenerative mitral stenosis. Eur Heart J. 2019;40(01):513-517.
- [Google Scholar]
- Infiltrative cardiovascular diseases: cardiomyopathies that look alike. J Am Coll Cardiol. 2010;55(17):1769-1779.
- [Google Scholar]
- Dilated cardiomyopathy with conduction disease and arrhythmia. Circulation. 2010;122(05):527-534.
- [Google Scholar]
- Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail. 2018;20(12):1713-1720.
- [Google Scholar]
- Right ventricular sarcoidosis: is it time for updated diagnostic criteria? Tex Heart Inst J. 2014;41(02):203-207.
- [Google Scholar]
- A simple voltage/mass index improved diagnosis of cardiac amyloidosis; an electrocardiographic and echocardiographic study of 570 patients with left ventricular hypertrophy. J Am Coll Cardiol. 2012;59:E1586.
- [Google Scholar]
- Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22(03):147-155.
- [Google Scholar]
- High prevalence of intracardiac thrombi in cardiac amyloidosis. J Am Coll Cardiol. 2019;73(13):1733-1734.
- [Google Scholar]
- Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging. 2017;10(04):398-407.
- [Google Scholar]
- Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PLoS One. 2014;9(12):e115910.
- [Google Scholar]
- Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14(03):205-212.
- [Google Scholar]
- Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(02):736-755.
- [Google Scholar]
- Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(07):624-632.
- [Google Scholar]
- Sarcoidosis in India: a review of 125 biopsy-proven cases from eastern India. Sarcoidosis. 1990;7(01):43-49.
- [Google Scholar]
- 1998. p. :472-480. Sarcoidosis: an Indian perspective. In: Das AK, ed. Postgraduate Medicine. Bombay: Association of Physicians of India
- Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Annu Rev Med. 2020;71:203-219.
- [Google Scholar]
- Standardization of 99mTechnetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol. 2018;25(01):181-190.
- [Google Scholar]
- Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11(01):e007030.
- [Google Scholar]
- Cardiac MR imaging of hypertrophic cardiomyopathy: techniques, findings, and clinical relevance. Magn Reson Med Sci. 2018;17(02):120-131.
- [Google Scholar]
- Role of cardiac MRI in assessment of patients with dilated cardiomyopathy. The Egyptian J Radiol Nuclear Med. 2017;48(04):853-860.
- [Google Scholar]
- NAME OF COLLAB GROUP. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med. 2017;58(08):1341-1353.
- [Google Scholar]
- (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6(02):195-201.
- [Google Scholar]
- Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119(02):167-172.
- [Google Scholar]
- Clinical characteristics, pulmonary function abnormalities and outcome of prednisolone treatment in 106 patients with sarcoidosis. J Assoc Physicians India. 2001;49:697-704.
- [Google Scholar]
- Sarcoidosis: global scenario & Indian perspective. Indian J Med Res. 2002;116:221-247.
- [Google Scholar]
- American Heart Association; American College of Cardiology; European Society of Cardiology; Heart Failure Society of America; Heart Failure Association of the European Society of Cardiology; Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. J Am Coll Cardiol. 2007;50(19):1914-1931.
- [Google Scholar]
- Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(02):e000098.
- [Google Scholar]
- Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014-1020.
- [Google Scholar]
- A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799-2806.
- [Google Scholar]
- Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47(02):355-374.
- [Google Scholar]
- ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016.
- [Google Scholar]






