Translate this page into:
Post-Glenn Shunt Systemic Desaturation—Is Venovenous Collateral the Culprit?
Hemanth Harish Ponnana Department of Cardiology, Nizam’s Institute of Medical Sciences (NIMS) Punjagutta, Hyderabad, Telangana 500 082 India hemu2030@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
After classic Glenn shunt or bidirectional cavopulmonary anastomosis, the reappearance or deepening of cyanosis may be due to systemic venous collateral channels. There are only few case reports on this issue in the present literature. Here, we present two cases that underwent bidirectional Glenn shunt, who later presented with cyanosis and desaturation and both of them were found to have venovenous collaterals.
Keywords
venovenous collaterals
glenn shunt
pericardioprenic collaterals
intercostobronchial collaterals
Introduction
In univentricular heart physiology, bidirectional cavopulmonary anastomosis (BCPA) is done as intermediate or palliative procedure1 before total correction by Glenn. The main aim is to allow low continuous blood flow to pulmonary arteries which causes the better growth of the pulmonary arteries, and simultaneously decreases the volume load on the functional single ventricle.2 3 4
In order to fill the venous atrium, pulmonary artery pressure should be more than venous atrium to overcome the pulmonary vascular resistance which, in turn, increases the superior vena cava (SVC) pressure.5 6 The difference in the SVC and inferior vena cava (IVC) pressure open up the venous collaterals to decompress SVC pressure3 7 8 9 which, in turn, decreases the pulmonary blood flow and increases the systemic desaturation. Sometimes, such extensive collaterals cause venous steal diverting unsaturated blood to go to IVC and then to the heart without going to the lung, producing severe cyanosis, and requires closure of the collaterals to improve the symptomatic status of the patient.
Here, we present two case reports of such patients with venovenous channels in cyanotic congenital heart disease post-Glenn shunt and would like to highlight the importance of possessing knowledge regarding the incidence and predilection site of systemic venous collaterals as well as therapeutic options.
Case Report
Case Report 1
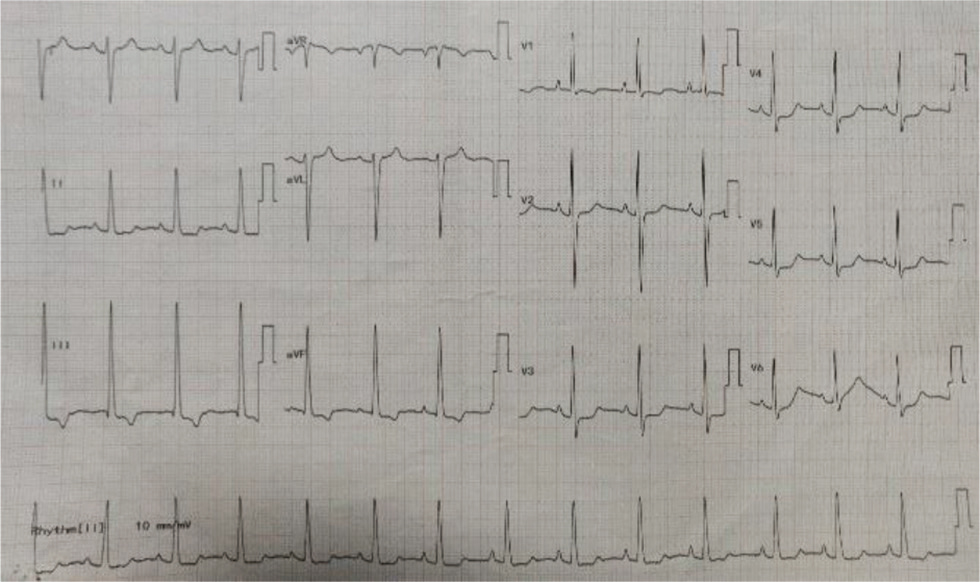
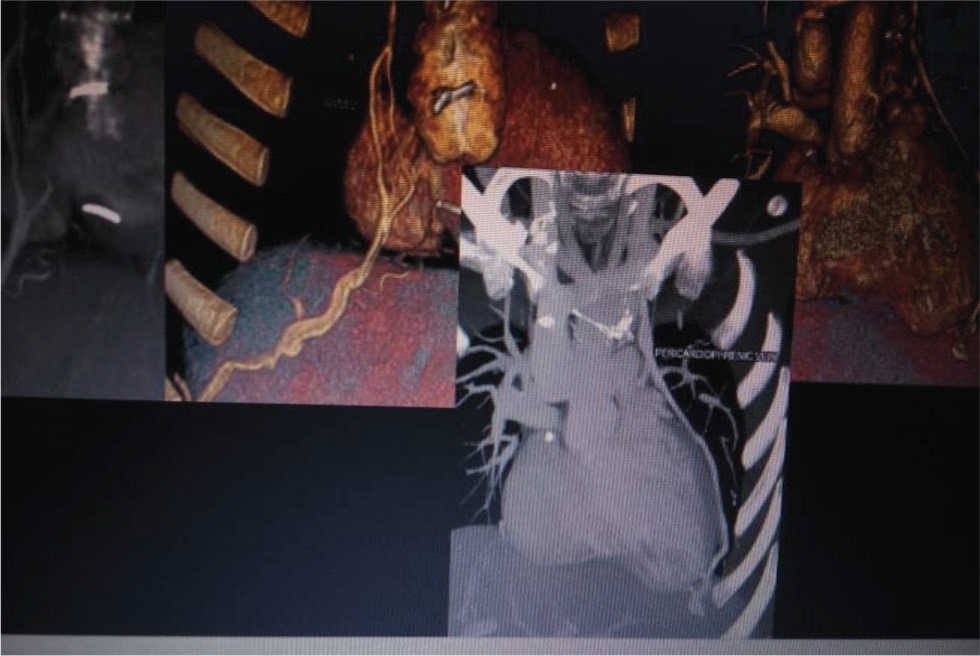
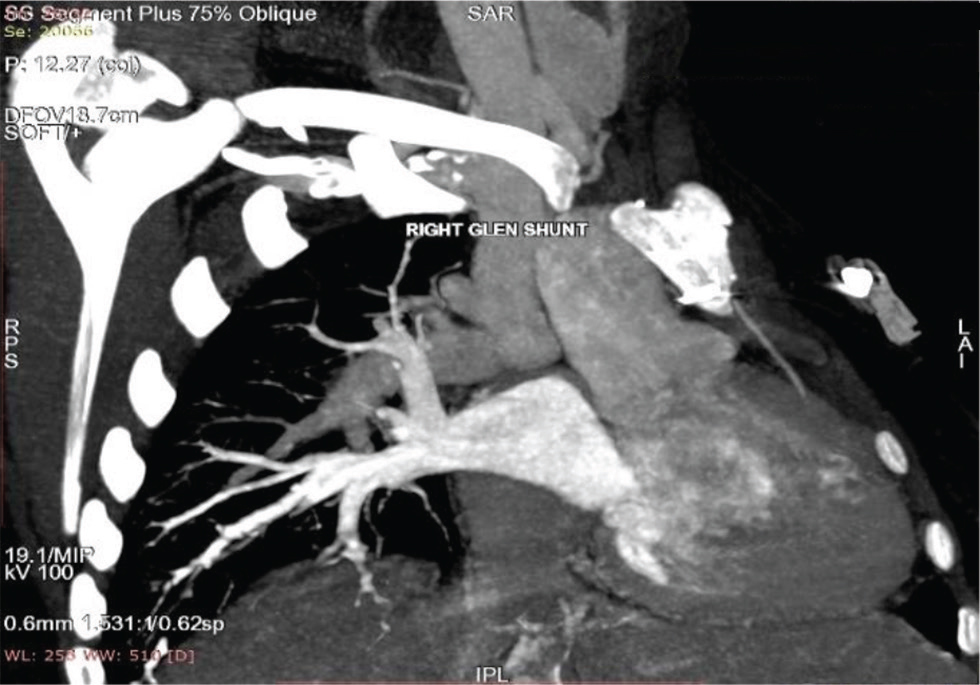
Our first patient is a 19-year-old female presented at 4 years of age to our center with history of NYHA Class II shortness of breath associated with central cyanosis and history of squatting episodes. Evaluation at that time showed that she had cyanotic congenital heart disease, single ventricle of left ventricular (LV) morphology with normally related great arteries, severe PS with good-sized confluent pulmonary arteries, and multiple major aortopulmonary collateral arteries (MAPCAS) not in failure. She underwent bidirectional Glenn shunt on November 28, 2005, for the same with a plan to undergo Fontan at a later date, following which she was on regular follow-up at our center. Presently, she came with the complaints of intermittent episodes of atypical chest pain of 1-year duration and progressive exertional shortness of breath of 6-months duration. There is history of two episodes of syncope in the last 6 months not associated with any abnormal muscle movements and involuntary passing of urine or stool and there was no history of post ictal drowsiness. On general examination, she had Grade 3 pan digital clubbing with central cyanosis and normal jugular vein pressure (JVP). Cardiovascular (CVS) examination showed apex beat localized to left fourth intercostal space (ICS), 1 cm lateral to midclavicular line, right ventricular (RV) type heaving apex with prominent pulsations in second left ICS; on auscultation, there is single S2 with ejection systolic murmur across pulmonary valve. Her electrocardiogram showed normal sinus rhythm with rates of 84 beats per minute, right axis deviation with counterclockwise loop, right atrial enlargement, and right ventricular hypertrophy (RVH) with strain pattern and narrow QRS (Fig. 1). Chest X-ray PA view showed well-centralized, well-exposed film with cardiomegaly and RV type apex, concave pulmonary bay, and oligemic lung fields (Fig. 2). Two-dimensional (2D) echo showed features suggestive of single ventricle with LV morphology and severe pulmonary stenosis, good functioning bidirectional Glenn shunt, and good LV function. She underwent cardiac catheterization on December 14, 2019, which showed cyanotic congenital heart disease, single ventricle with LV morphology, side by side great arteries, severe PS with good-sized confluent pulmonary arteries with normal LV, RV, and aortic pressures, and decreased PA pressures not in failure. However, when we took a left brachial vein injection, we found pericardiophrenic collateral opening into right atrium (Fig. 3). She underwent CT thoracic aortogram which showed situs solitus, D Loop, levocardia, side by side arteries with severe pulmonary stenosis, and hypoplastic main pulmonary artery (MPA) (16.5 mm), right pulmonary artery (RPA) (16 mm), and left pulmonary artery (LPA) (14.5 mm) along with pericardiophrenic venovenous collateral (Fig. 4) and multiple small intercostobronchial collaterals in the mediastinum. CT thoracic aortogram also showed bidirectional Glenn shunt (Fig. 5).
-
Fig. 1 ECG of 19-year-old girl showing right axis deviation with counterclockwise loop, right atrial enlargement, RVH with strain pattern, and narrow QRS. RVH, right ventricular hypertrophy.
Fig. 1 ECG of 19-year-old girl showing right axis deviation with counterclockwise loop, right atrial enlargement, RVH with strain pattern, and narrow QRS. RVH, right ventricular hypertrophy.

-
Fig. 2 Chest X-ray of 19-year-old girl shows well-centralized, well exposed film showing cardiomegaly, RV type apex, and concave pulmonary bay with oligemic lung fields.
Fig. 2 Chest X-ray of 19-year-old girl shows well-centralized, well exposed film showing cardiomegaly, RV type apex, and concave pulmonary bay with oligemic lung fields.

-
Fig. 3 Cardiac catheterization of 19-year-old girl showing venovenous collateral pericardiophrenic vein opening into right atrium, causing desaturation and cyanosis of the patient.
Fig. 3 Cardiac catheterization of 19-year-old girl showing venovenous collateral pericardiophrenic vein opening into right atrium, causing desaturation and cyanosis of the patient.
-
Fig. 4 Cardiac CT of 19-year-old girl showing pericardiophrenic venous collateral opening into right atrium.
Fig. 4 Cardiac CT of 19-year-old girl showing pericardiophrenic venous collateral opening into right atrium.
-
Fig. 5 Cardiac CT scan of 19-year-old girl showing right-sided bidirectional Glenn shunt, and SVC connecting right pulmonary artery. SVC, superior vena cava.
Fig. 5 Cardiac CT scan of 19-year-old girl showing right-sided bidirectional Glenn shunt, and SVC connecting right pulmonary artery. SVC, superior vena cava.
Case Report 2
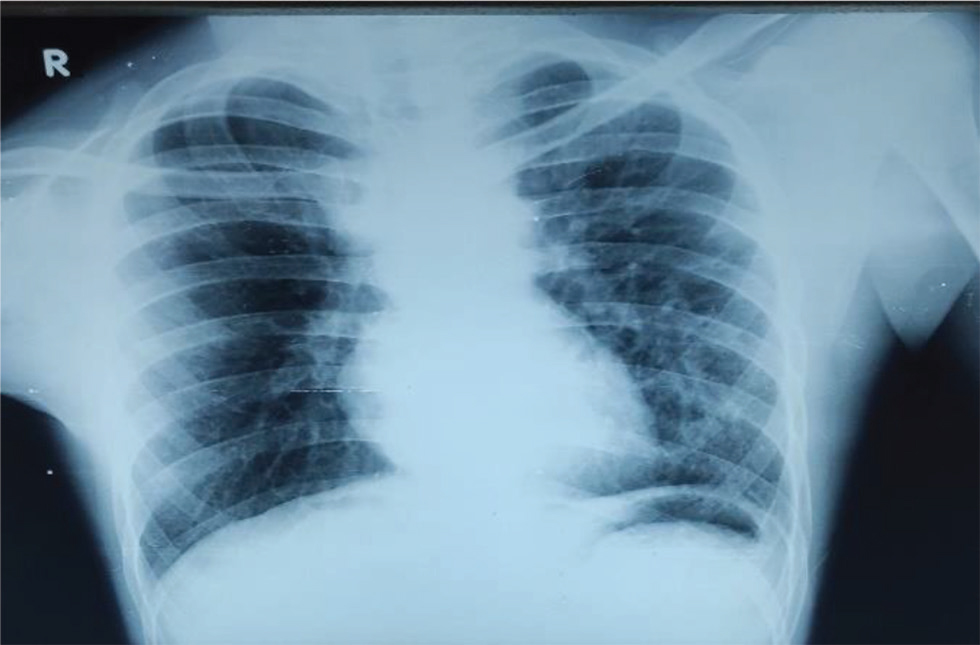
Our second patient is a 16-year-old male patient who presented with complaints of shortness of breath of functional class III with cyanosis. He was presented at the age of 18 months with complaints of cyanosis, shortness of breath, with squatting episodes since the time he started walking, and poor weight gain. Subsequently, he was diagnosed to have cyanotic congenital heart disease, tetralogy of Fallot with large subaortic ventricular septal defect (VSD), and good confluent pulmonary arteries. He underwent bidirectional Glenn shunt with a plan to undergo Fontan at a later date. During the same time, he had cerebrovascular accident (left middle cerebral artery territory infarct) with residual weakness and wasting of right upper and lower limbs. During present admission, general examination showed Grade 3 pan digital clubbing with central cyanosis and normal JVP. CVS examination showed RV type heaving apex localized to left fourth ICS 1 cm lateral to midclavicular line (MCL). On auscultation, there was an ejection systolic murmur of across pulmonary valve. His electrocardiography (Fig. 6) showed normal sinus rhythm of rates 78 per minute, right axis deviation with clockwise loop, right atrial enlargement, RVH without strain pattern, and narrow QRS. Chest X-Ray PA (Fig. 7) showed well-centralized, well-exposed film with normal cardiac silhouette, RV type apex, concave pulmonary bay, and oligemic lung fields. 2D echo showed features suggestive of tetralogy of Fallot with bidirectional Glenn shunt, large subaortic VSD with right to left shunt, and severe pulmonary valvular stenosis with mean gradient of 42 mm Hg. He underwent cardiac catheterization on January 28, 2020 (Fig. 8), which showed gross systemic hypoxemia with normal femoral artery (FA), LV, right atrium (RA), left atrium (LA) and PA pressures with systemic RV pressures not in failure. Angiogram showed features suggestive of tetralogy of Fallot with large subaortic VSD and right to left shunt with hypoplastic MPA and stenotic LPA, functional bidirectional Glenn shunt, and presence of large pericardiophrenic venovenous collateral. He underwent CT aortogram on January 30, 2020, which showed right sided bidirectional Glenn shunt (Fig. 9) and pericardiophrenic collateral with multiple intercostobronchial collaterals (Fig. 10).

-
Fig. 6 ECG of 16-year-old boy showing right axis deviation with counterclockwise loop, right atrial enlargement, RVH without strain pattern, and narrow QRS. RVH, right ventricular hypertrophy.
Fig. 6 ECG of 16-year-old boy showing right axis deviation with counterclockwise loop, right atrial enlargement, RVH without strain pattern, and narrow QRS. RVH, right ventricular hypertrophy.
-
Fig. 7 Chest X-ray of 16-year-old boy shows well-centralized, well exposed film showing cardiomegaly, right ventricular type apex, and concave pulmonary bay with oligemic lung fields.
Fig. 7 Chest X-ray of 16-year-old boy shows well-centralized, well exposed film showing cardiomegaly, right ventricular type apex, and concave pulmonary bay with oligemic lung fields.
-
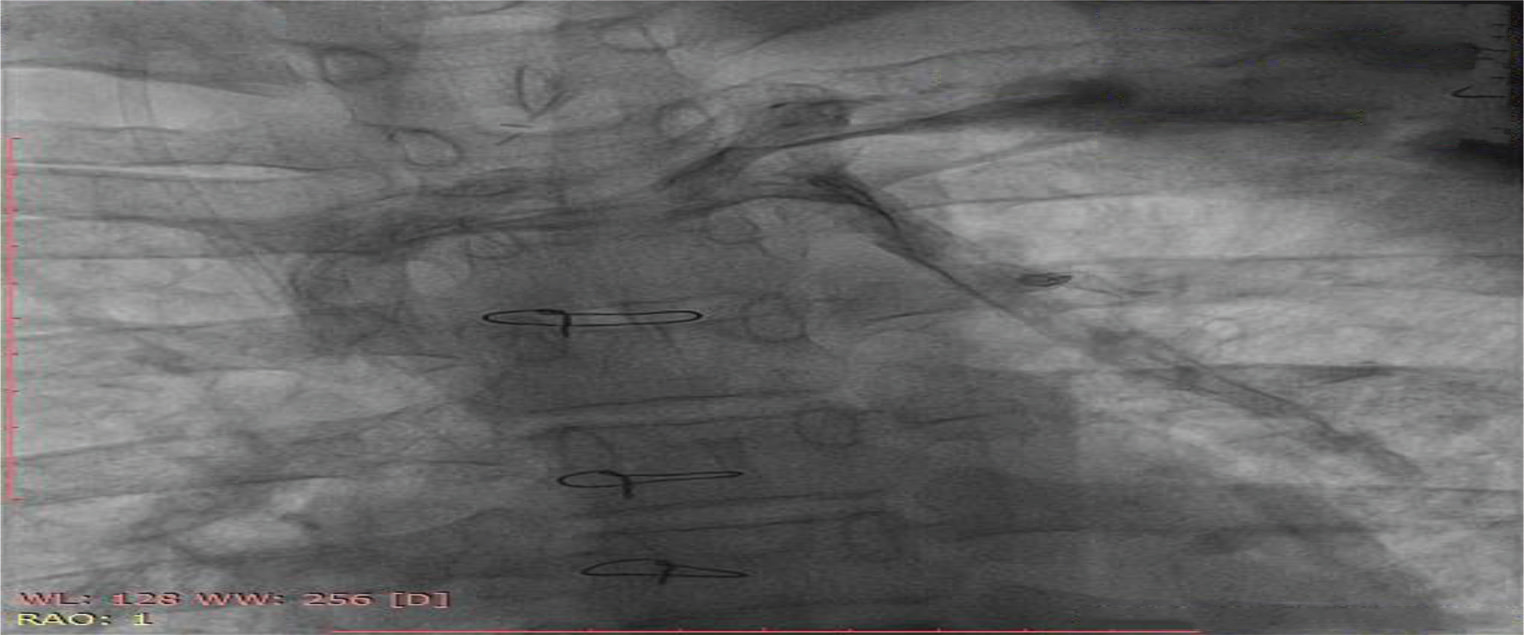
Fig. 8 Cardiac catheterization of 16-year-old boy showing venovenous collateral, causing desaturation and cyanosis of the patient.
Fig. 8 Cardiac catheterization of 16-year-old boy showing venovenous collateral, causing desaturation and cyanosis of the patient.
-
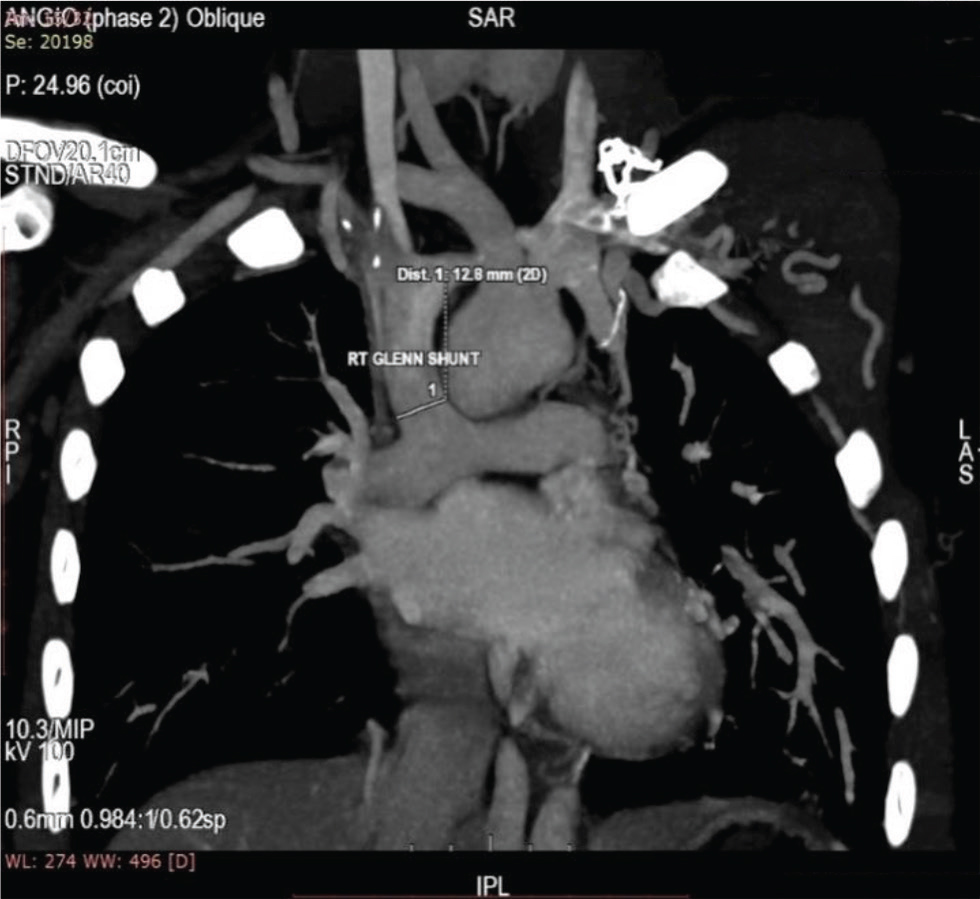
Fig. 9 CT aortogram of 16-year-old boy showing right-sided bidirectional Glenn shunt, and SVC connecting right pulmonary artery. SVC, superior vena cava.
Fig. 9 CT aortogram of 16-year-old boy showing right-sided bidirectional Glenn shunt, and SVC connecting right pulmonary artery. SVC, superior vena cava.
-
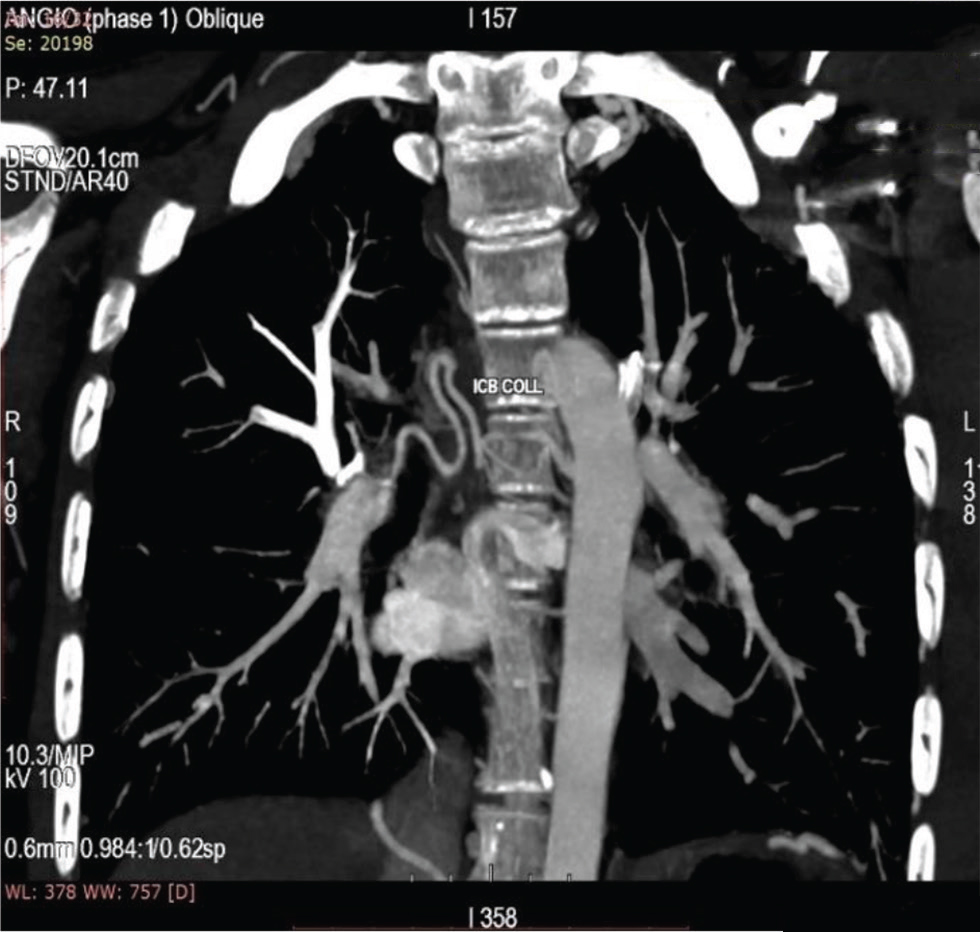
Fig. 10 CT aortogram of 16-year-old boy showing intercostobronchial collaterals opening into right atrium.
Fig. 10 CT aortogram of 16-year-old boy showing intercostobronchial collaterals opening into right atrium.
Discussion
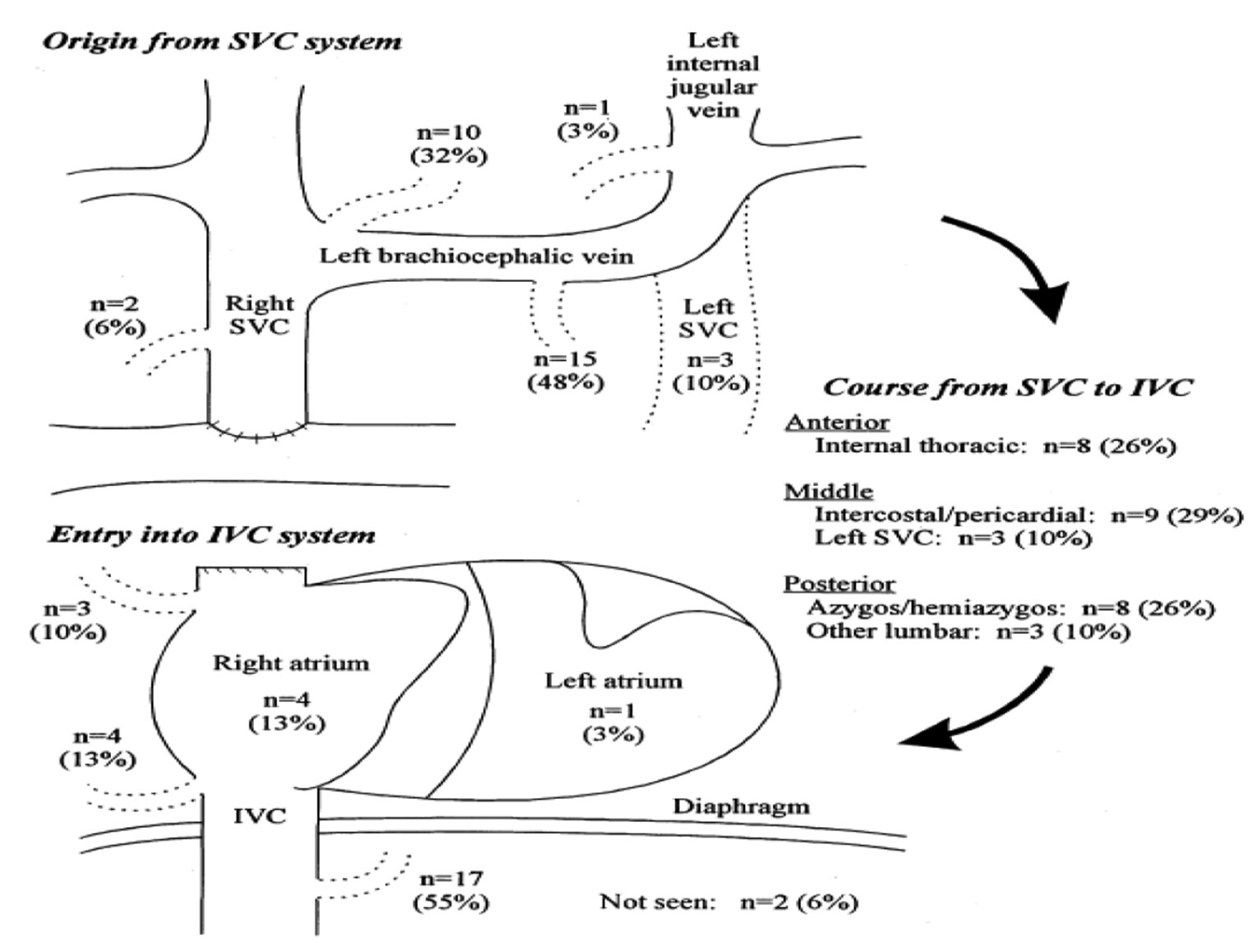
The human venous system mainly derived from three embryological roots, the umbilical veins of chorionic origin, omphalomesenteric veins, and cardinal veins. The cardinal veins are further divided into supracardinal and subcardinal veins. During development, all these venous systems unite to form the right and left horns of sinus venosus, which is the single common receiving chamber that opens into the primitive heart. All the complex embryonic changes in these systemic venous systems occur around 6 to 8 weeks of embryonic life under the influence of pressure and flow dynamics of cardiac growth. After all these changes, there will be a number of obliterated venous residues. Most important one of these venous residues is the ligamentum marshalli, which was the remnant of left SVC connecting with coronary sinus (CS). Normally, persistent LSVC and azygous continuation of an interrupted IVC are considered as normal variants; however, in case of univentricular physiology, these anomalies are responsible for significant patient mortality and morbidity. Various sites of collaterals opening between SVC and IVC are represented in Fig. 11.
-
Fig. 11 Figure showing various venovenous collaterals channels between superior vena cava and inferior vena cava.
Fig. 11 Figure showing various venovenous collaterals channels between superior vena cava and inferior vena cava.
Patients who had underwent Glenn and Fontan procedures are the ones who are prone for reopening of various previously occluded venous channels and this has been documented in literature. The main reason for the opening of these occluded venous channels is noted to be because of increased venous filling pressures. Patients with interrupted IVC, (hemi)azygous continuation, and intrahepatic veins directly open into the corresponding atrium. While doing BCPA or Glenn procedure, if the intrahepatic vessels are excluded, then it may later result in venous steal shunting blood away from the intended cavopulmonary circulation.
As an increasing amount of data is available about pulmonary circulation, either entirely or primarily dependent on BCPA, it has become clearer that most of the individuals with BCPA or Glenn manifest various vascular anomalies like pulmonary AV fistulas,10 aorta pulmonary collaterals,11 and venovenous collaterals. Among these, the significance of aorta pulmonary collaterals in the setting of BCPA is not known, whereas pulmonary AV fistulas and venovenous collaterals will cause life-threatening cyanosis and systemic desaturation.9 12
Increased systemic venous filling pressures, not very suitable hemodynamics and right ventricular anatomy are considered as risk factors for the development of systemic venous collaterals. Heterotaxy syndromes, particularly those with right isomerism (asplenia), are associated with significant development of venovenous collaterals. The most important reason for the development of systemic venovenous collaterals post-Glenn is because of increased pressure gradient between upper and lower half of the body, secondary to Glenn procedure. Hence, wherever feasible, early Fontan is considered against Glenn as a final procedure in the management of univentricular hearts. There is evidence from various fluid dynamic studies involving cavopulmonary anastomosis that very little differences in pressure can cause significant changes in energy conservation at cavopulmonary connections.13 14 Other factors responsible for the development of venovenous collaterals channels are propensity of the specific individuals to develop collaterals and patient’s native venous anatomy. Most of the times, the abnormal venovenous collaterals, considered abnormal,9 are secondary to patency and not because of their existence.
Systemic venovenous collaterals are common both in patients with heterotaxy syndromes, and also in patients afflicted with heterotaxy syndromes who had undergone BCPA. Due to this reason alone, the patients undergoing BCPA should be evaluated for presence of any SVC system anomalies. Even though only a small percentage of patients show these collaterals pre-BCPA, it is worth identifying them as these are the patients who develop early onset desaturation post-BCPA.
Another most important finding that is observed in previous studies as well as our study is the decreased or absent upper lobe pulmonary blood flow. It has been observed that the patients who had undergone BCPA or Glenn and developed venovenous collaterals showed decreased flow to the upper pulmonary lobes and the incidence is as high as 20%. This finding has been successfully reported by Trusler et al7 in their various case reports on BCPA and Glenn anastomosis.
Development of systemic desaturation is considered as one of the dreaded complications post-BCPA or post-Glenn procedure. In our case series, and also in many studies before, it has been noted that development of venovenous channels is considered the main cause for the development of desaturation post-BCPA or post-Glenn. Hence, with regard to the development of desaturation early or late in a patient who had undergone BCPA or Glenn, we must consider development of venovenous collaterals. Even though there are significant differences in saturations among various patients, there is no specific threshold which could help in identifying patients with venovenous collaterals.
With respect to therapeutic interventions, coil embolization and occluding of the unwanted venous channels15 is the most preferred because of its minimally invasive nature; moreover, most of the times it can be combined with diagnostic procedure. Among all percutaneous procedures, coil embolization is the most commonly used and is associated with considerable success rates. There is increase in saturations up to approximately 20%.
Most patients undergoing BCPA or Glenn have that procedure done as a palliative procedure with requirement of Fontan at a later date. Hence, once Fontan is completed, the significance of these venovenous collaterals draining below the diaphragm are minimal. Hence, addressing these venovenous collaterals should be on patient to patient basis and also based on circumstances. There is no need to embolize patients with mild cyanosis and asymptomatic patients post-BCPA or post-Glenn if Fontan is being planned. However, the venous collaterals opening into LA should be addressed at the time of diagnosis or during Fontan when they are diagnosed. Whenever feasible better to opt for coil embolization than surgical ligation. All other patients with venovenous collaterals above the diaphragm should be either embolized or surgically ligated whenever they are diagnosed. Patients who had undergone BCPA or Glenn and venovenous collaterals were suspected, treatment either by embolization or surgery should be based on symptoms. In patients with significant symptoms and desaturation, diagnostic catheterization followed by embolization or surgery is usually done.
Based on the knowledge from our case reports and also previous studies, it is imperative to think that Fontan, wherever feasible, should be the primary procedure of choice. Whenever it is not feasible and either BCPA or Glenn is planned before Fontan completion, we should have high-index of suspension for development of venovenous collaterals and look for any evidence of desaturation both early and late. In symptomatic patients with significant desaturation, either go for a Fontan completion, coil embolization, or surgical ligation of collaterals. In our case, we sent both our patients for surgical ligation followed by, if possible, completion of Fontan.
Conclusion
Finally, from the case series we presented we can conclude that treating doctors should have knowledge about the probable complications that can happen following a BCPA or Glenn procedure. Whenever a patient becomes symptomatic and develops significant desaturation post-BCPA or post-Glenn, the treating cardiologist should have high-index of suspicion to look for development of any systemic venovenous collaterals. Treating doctor should have accurate knowledge about possible connections between SVC and IVC and should meticulously look at those locations for the development of any venovenous collaterals. Whenever possible we should look for SVC anomalies before the patient undergoes planned BCPA or Glenn. As in our two cases, late presentation of desaturation and development of venovenous collaterals, Fontan completion is indicated when pulmonary hemodynamics are favorable, otherwise we should plan to close the collaterals either by embolization or surgical ligation. Hence, a high-index of suspicion for looking out whether venovenous collaterals are present, when a patient presents with desaturation and cyanosis following BCPA or Glenn, and managing them accordingly is of paramount importance.
Conflict of Interest
None declared.
References
- Primary bidirectional superior cavopulmonary shunt in infants between 1 and 4 months of age. Ann Thorac Surg. 1995;59(05):1120-1125, discussion 1125–1126.
- [Google Scholar]
- Physiological rationale for a bidirectional cavopulmonary shunt. A versatile complement to the Fontan principle. J Thorac Cardiovasc Surg. 1985;90(03):391-398.
- [Google Scholar]
- bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation. 1990;82(05):IV170-IV176.
- [Google Scholar]
- Usefulness of the bidirectional Glenn procedure as staged reconstruction for the functional single ventricle. Am J Cardiol. 1993;71(11):959-962.
- [Google Scholar]
- Further experiments on long term survivors after circulatory bypass of the right side of the heart. Surg Gynecol Obstet. 1964;119:302-310.
- [Google Scholar]
- Long-term evaluation of cava-pulmonary artery anastomosis. Surgery. 1973;74(06):899-916.
- [Google Scholar]
- William Glenn lecture. The cavopulmonary shunt. Evolution of a concept. Circulation. 1990;82(05):IV131-IV138.
- [Google Scholar]
- Increasing cyanosis early after cavopulmonary connection caused by abnormal systemic venous channels. Br Heart J. 1995;73(02):182-186.
- [Google Scholar]
- Development of pulmonary arteriovenous fistulae in children after cavopulmonary shunt. Circulation. 1995;92(09):II309-II314.
- [Google Scholar]
- Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22(01):207-215.
- [Google Scholar]
- Fulminant development of pulmonary arteriovenous fistulas in an infant after total cavopulmonary shunt. Pediatr Cardiol. 1996;17(01):46-50.
- [Google Scholar]
- Use of computational fluid dynamics in the design of surgical procedures: application to the study of competitive flows in cavo-pulmonary connections. J Thorac Cardiovasc Surg. 1996;111(03):502-513.
- [Google Scholar]
- Comparison by computerized numeric modeling of energy losses in different Fontan connections. Circulation. 1995;92(09):II322-II326.
- [Google Scholar]
- Coil embolization to occlude aortopulmonary collateral vessels and shunts in patients with congenital heart disease. J Am Coll Cardiol. 1989;13(01):100-108.
- [Google Scholar]







