Translate this page into:
Mechanical Prosthetic Valve Thrombus in a Term Pregnant Woman Presenting as Acute Heart Failure: Case Report and Review of Literature
Sasirekha Rengaraj Department of Obstetrics and Gynaecology, Jawaharlal Institute of Postgraduate Medical Education and Research Puducherry 605006 India sasirekha0226@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Cardiovascular disease in pregnancy contributes to a significant proportion of death worldwide. Though pregnancy-associated myocardial infarction and aortic dissection are the common causes of adverse cardiac events in developed countries, rheumatic heart diseases continue to be the important reason for cardiovascular morbidity and mortality in developing countries. The risk of adverse cardiac outcome is dependent on the type and severity of valvular abnormality, functional status, left ventricular function, and pulmonary arterial pressure. Managing a pregnant woman with a mechanical heart valve prosthesis is challenging because of the difficulty in achieving optimal anticoagulation in the presence of hypercoagulability. Mitral valve thrombus is a life-threatening event and women can present with acute heart failure or thromboembolic events. We report successful management of a 26-year-old primigravida with rheumatic heart disease diagnosed to have huge thrombus on mechanical prosthetic mitral valve presented with acute heart failure at 36 weeks. She received multidisciplinary care and underwent concurrent cesarean section followed by thrombectomy under cardiopulmonary bypass. She had a good recovery following surgery and the complexity surrounds the management merit the presentation with a review of management strategies for a women with mechanical prosthetic heart valve in pregnancy.
Keywords
mechanical prosthetic heart valve
vavle thrombus
mitral stenosis
pregnancy
Introduction
Cardiovascular disease in pregnancy contributes to significant proportion of deaths worldwide. Though pregnancy-associated myocardial infarction (PAMI) and aortic dissection are the common causes of adverse cardiac events in developed countries, rheumatic heart diseases (RHDs) continue to be the important reason for cardiovascular morbidity and mortality in emerging countries.1 2 The risk of adverse cardiac outcome is dependent on the type and severity of valvular abnormality, functional status, left ventricular function, and pulmonary arterial pressure.2 3 Managing a pregnant woman with mechanical prosthetic heart valve (MPHV) is challenging because of the difficulty in achieving optimal anticoagulation in the presence of hypercoagulability. Due to different maternal and fetal risks associated with anticoagulation, along with absence of randomized controlled trials, the optimal anticoagulation regimen in pregnant women remains uncertain. MPHV thrombus is a life-threatening event and women can present with acute heart failure or thromboembolic events. We report a management of 26-year-old primigravida with RHD diagnosed to have huge thrombus on mechanical prosthetic mitral valve presented with acute heart failure at 36 weeks. She received multidisciplinary care and underwent concurrent cesarean delivery followed by thrombectomy under cardiopulmonary bypass (CPB). She had a good recovery following surgery and the complexity surrounds the management merit the presentation with review of management strategies of MPHV in pregnancy.
Case Details
A 24-year-old primigravida, known case of MPHV on unfractionated heparin (UH), presented to emergency department with complaints of acute onset of shortness of breath and dizziness for 1 day. She underwent MPHV replacement for RHD with severe mitral stenosis at 15 years of age. This was TTK Chitra prosthetic valve which is a type of tilting disc valve and requires therapeutic anticoagulation to maintain target international normalized ratio (INR) of 2.5 to 3. Following that, she was taking oral vitamin K antagonist (VKA), warfarin and was on regular follow-up till marriage. The valve function was satisfactory and she was on warfarin 3 mg a day. She conceived spontaneously a year after marriage. She had no preconceptional visits, continued warfarin 3mg during first trimester and had consulted for the first time at 16 weeks of gestation. The INR was within the target range. Her cardiac evaluation showed well-functioning prosthetic valve. The target fetal scan at 18 weeks was normal including fetal echo at 22 weeks. She had regular antenatal and cardiology visits till 34 weeks. Her last INR prior to admission with us was 2.1 done at 32 weeks. She was switched over to UH at 34 weeks and was on 5000 units subcutaneously three times a day for past 10 days, and the activated partial thromboplastin time report was not available. She was referred at 36 weeks to our center with symptoms and signs of acute heart failure.
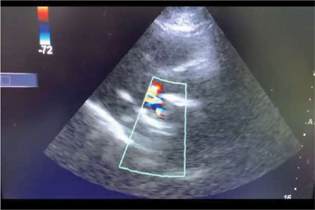
At admission, she was stable with respiratory rate of 28/minute and her functional status was New York Health Association class III. Her PR pulse rate was 116/minute with normal sinus rhythm. The blood pressure was 100/70 mm Hg. She also complained of chest pain and her electrocardiogram was normal. The oxygen saturation was 94% on room air. On auscultation, the valve click was muffled in addition to fine crepitations on lower lung fields on both the sides. She was not in labor and the fetal condition was reassuring. Urgent call was sent to cardiologist with the suspicion of MPHV thrombus. Transthoracic echocardiogram (TTE) showed severe restricted mitral prosthetic valve movement with a thrombus of 1.9 × 1.4cm attached to ventricular aspect of mitral prosthetic valve (Fig. 1). The peak gradient across the mitral valve was 54/40 mm Hg with right ventricular systolic pressure of 70 mm Hg. There was severe aortic regurgitation (AR) and tricuspid regurgitation (TR). No vegetation or pericardial effusion. She was started on UH of 5000u intravenously every 6th hourly. After multidisciplinary discussion, emergency concurrent cesarean section and thrombectomy/valve replacement were planned. The family was made aware of the plan including the need for intensive care unit (ICU) stay in the postoperative period.
-
Fig. 1 Echo cardiac image of prosthetic mitral valve thrombus.
Fig. 1 Echo cardiac image of prosthetic mitral valve thrombus.
Under general anesthesia, emergency caesarean section was done first and delivered an alive, borderline term, small for gestational age boy baby with 5-minute APGAR of eight. Prophylactic oxytocin was started and it was ensured that the uterus was contracted and retracted well with assurance of optimal hemostasis before closure. Then the cardiothoracic surgery team took over, proceeded with redo sternotomy. Adhesions were released. After aortobicaval cannulation and establishment of CPB, left atrium (LA) was opened by classic LA approach. Prosthetic mitral valve was inspected and the thrombus on MHPV was removed (Fig. 2). The thrombus on ventricular aspect could not be approached through this route, hence aorta was opened. The thrombus on left ventricular aspect was removed completely. After through wash, the valve function was observed and its opening was satisfactory. Hypothermia was reversed. Gradual incremental load to heart was given before the CPB was weaned off. The cardiac function was noted to be satisfactory and she was on moderate doses of inotropes. She was extubated on the next day electively and required an ICU stay for 48 hours. She was on oral acenocoumarol 3 and 2 mg per day alternatively and the optimal INR was achieved. She was discharged after 7 days of hospital stay and the baby was doing well. She was advised to do weekly INR till it was stabilized. In addition, she was on tab. aspirin 75 mg a day, minimal doses of oral metoprolol, furosemide, and enalapril. When she came for postnatal check-up after 6 weeks, Cu-T intrauterine device was inserted. Currently, she is doing well and on regular follow-up. Postoperative follow-up transthoracic echo showed moving prosthetic valve and mild AR/TR.

-
Fig 2 Intraoperative picture showing thrombus in mechanical mitral valve.
Fig 2 Intraoperative picture showing thrombus in mechanical mitral valve.
Discussion
Cardiac interventions in the setting of severe RHD are quite successful and include a variety of surgeries ranges from percutaneous procedures to interventions requiring CPB. Though the surgical procedures are possible during pregnancy, optimizing cardiac conditions before becoming pregnant are extremely important. Generally, whenever valve replacement is needed in young women, MPHV is preferred over bioprosthetic valve because of durability and its ability to withstand the hemodynamics of pregnancy.2 3 4 However, they need lifelong anticoagulation with oral VKA.
The hypercoagulable state of pregnancy and increase in blood volume necessitate the need for higher doses of anticoagulation and frequent monitoring. The risk of thromboembolic complications (TEC) is more during pregnancy as there is a complexity in achieving optimal anticoagulation. The MPHV used nowadays like tilted and leaflet valves possess lower risk of TEC, but choosing appropriate anticoagulation regime is difficult because of lack of strong evidence.2 The risk of valvular and extravalvular TEC depends on the type of anticoagulation regimen and ranges from 2.7 to8.7% in the recent meta-analysis.5 6 As per modified World Health Organization (mWHO) classification, MPHV is grouped under class III and the risk of adverse cardiac event rate is 19 to 27%.3 The occurrence of serious complication of MPHV thrombus was 4.7% in a study by Registry of Pregnancy and Cardiac Disease (ROPAC) among 212 patients on MPHV. The ROPAC study also highlighted the possibility of uncomplicated pregnancy with a live birth with MPHV was only 58% when compared with patients on biprosthetic valve (n = 134) and patients without prosthetic valve (n = 2620). The risk of hemorrhagic complications while on anticoagulant was 23% in their study.3 Both TEC and hemorrhagic complications are increased in women with MPHV, the TEC are especially associated with mortality. These hemorrhagic complications are more in developed countries due to increase in caesarean deliveries and differences in anticoagulation use.2 3 5 In general, the TEC are more in mitral valve replacements than in aortic valve replacements, but in ROPAC study, the difference was not found to be significant (4.4 vs. 2.6%, p = 1.00).2 3
There are three different approaches regarding anticoagulation regimens for managing women with MPHV. The options include (i) VKA throughout pregnancy, (ii) low molecular weight heparin (LMWH) throughout pregnancy, (iii) sequential administration of LMWH in first trimester followed by VKA in second and third trimester and LMWH/UH around delivery.7 8 Various studies and reviews have shown that there is a certain element of either maternal or fetal risk with each method and no particular regimen is superior to another. The risk of fetal loss including miscarriage, embryopathy, and fetal death increases when the woman is on VKA throughout the pregnancy.2 3 4 5 6 The usage of VKA in first trimester was associated with greater risk of miscarriage compared with heparin (28.6 vs. 9.2% p < 0.001). Unlike heparin, there is a risk of fetoplacental transfer with VKA; however, the fetal effects are dose dependent. The risk is highest when the women are on >5 mg of warfarin during the organogenesis period. It is known as warfarin embryopathy and characterized by nasal hypoplasia, lower birth weight, malformed bones, stippled epiphysis and deafness and the overall risk of embryopathy is <10% in first trimester.2 In terms of fetal outcome, heparin as an anticoagulant looked safer but the risks of TEC are more in women on heparin throughout.3 5
Of the 10 women who had MPHV thrombus in ROPAC study, five of them developed in first trimester and in all of them warfarin was switched over to LMWH. These TEC are more in a study by Vause et al who studied 58 women prospectively with MPHV during pregnancy and 71% of them used LMWH throughout the pregnancy.3 4 Of the five women died (9%), four of them had fatal MPHV thrombus and one woman had cerebrovascular accident (CVA). Out of 58 women studied, a total of 16% had either valve thrombus or valve dysfunction and 9% had CVA. The rate of these complications was more in the study by Vause et al that used LMWH predominantly (71%) compared with ROPAC (6.1%) and recent two systematic reviews (13.9% and 6.7%) in which the most common anticoagulant regimen was either VKA throughout or LMWH in first trimester and VKA in second and third trimesters.3 4 5 6 The TEC are commonly associated with subtherapeutic levels of anticoagulation and regular monitoring of anti-Xa levels is mandatory when the woman is on LMWH as the levels are found to be variable with physiological changes of pregnancy. There are no recommendations regarding the timing and frequency of monitoring anti-Xa levels and variations are noted in routine practice. Some recommend testing of the predose (trough) levels and some recommend 4 to 6 hours after the dose. However, all the recommendations strongly discourage LMWH, if facilities for monitoring anti-Xa levels are not available.5 7 8 Table 1 describes the summary of various guidelines for the management of pregnant women with MPHV.
|
Guidelines and year of update |
First trimester (6–12 weeks) |
Second and third trimester |
After 36 weeks |
Salient features |
|---|---|---|---|---|
|
ACC/AHA 2017 update of 2014 focused guideline |
Continue VKA if warfarin dose is <5 mg to achieve optimal INR (Or) LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose (Or) Dose-adjusted intravenous UH |
Oral warfarin (Or) LMWH twice daily (VKA is strongly recommended) |
LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose (Or) Dose-adjusted intravenous UH |
No subcutaneous UH Target INR for oral warfarin is 3 (2.5–2.5) Target anti-Xa level for LMWH is 0.8–1.2 U/mL For dose-adjusted intravenous UH, the target aPTT is twice that of normal Dose-adjusted intravenous UH is difficult in first trimester, as hospitalization is needed |
|
ACCP 2012 guideline |
VKA, oral warfarin if high risk of thromboembolism (Or) LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose (Or) Dose-adjusted subcutaneous UH |
VKA, oral warfarin (Or) LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose (Or) Adjusted dose UH subcutaneously throughout pregnancy |
LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose (Or) Dose-adjusted intravenous UH Change to UH 36 hours prior to labor |
Anticoagulant management to be individualized Target anti-Xa level for LMWH is 0.35–0.7 U/mL For dose-adjusted intravenous UH, the target aPTT is twice that of normal Target INR is 2–3 without high risk factors In women at very high risk for thromboembolism, VKA is acceptable (warfarin) throughout pregnancy Addition of aspirin is recommended for women at high risk of thromboembolism Frequent monitoring of anti-Xa level is needed, probably weekly |
|
ESC 2018 guideline |
Continue VKA if warfarin dose is <5 mg to achieve optimal INR (Or) LMWH twice daily with regular monitoring of anti-Xa level 4 to 6 hours after the dose (Or) Dose-adjusted intravenous UH |
VKA, oral warfarin (Or) LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose |
LMWH twice daily with regular monitoring of anti-Xa level 4–6 hours after the dose (Or) Dose-adjusted intravenous UH Change to UH 36 hours prior to labor and restart 4–6 hours after delivery if no bleeding |
Preferred anticoagulant in second and third trimester is VKA Target anti-Xa level for LMWH is 0.35–0.7 U/mL For dose-adjusted intravenous UH, the target aPTT is twice that of normal aPTT and anti-Xa monitoring weekly and INR every 2 weekly Changeover of anticoagulants should happen in hospital |
|
CSI 2019 guideline |
Continue VKA if patients are on minimal doses of VKA (Or) Heparin may be considered if warfarin dose is >5 mg or acenocoumarol >3 mg to achieve optimal INR |
VKA, oral warfarin |
If cesarean section is planned VKA is stopped 3 days before surgery If vaginal delivery is expected, VKA to be stopped at 34 weeks and LMWH to be started with close monitoring of anti-Xa levels Elective labor induction at 36–37 weeks |
Patients can be maintained on VKA if they are on minimal dose (warfarin <5 mg and acenocoumarol <3 mg) Regular anti-Xa monitoring is needed if they are on LMWH If aspirin is added in first trimester, the target INR can be reduced by 0.5 |
Abbreviations: ACC/AHA, American Society of Cardiology/American Heart Association; ACCP, American College of Chest Physicians; aPTT, activated partial thromboplastin time; ESI, European Society of Cardiologists; CSI, Cardiologist Society of India; INR international normalized ratio; LMWH, low molecular weight Heparin; UH, unfractionated heparin; VKA, vitamin K antagonist.
The management of MPV thrombosis depends on the size of thrombus, maternal stability, fetal viability, and valve function. Anticoagulant with UH is preferred if mother is stable and MPHV thrombus is <1 cm as there is increased fetal loss with CPB.2 3 9 If the mother is hemodynamically unstable or large obstructive thrombus, redo surgery in the form of either thrombectomy or valve replacement is preferred. Thrombolysis with intravenous tissue plasminogen activator is preferred if mother is hemodynamically unstable and surgery is contraindicated. Thrombolysis can safely be used in pregnancy as there are no fetal risks; however, it has increased risk of bleeding and TEC up to 10%. On the contrary, surgery carries increased fetal loss up to 30% and maternal risk of 5%.2 3 10
MPHV thrombus is often due to suboptimal anticoagulation and usually happens in the first and last trimester when VKA is switched over to heparin. Peripartum period is also a high-risk period as there is a huge change in cardiac hemodynamics around delivery. Our patient presented at 36 weeks and there was huge thrombus extending on to left ventricle; surgery was undertaken along with concurrent cesarean section. The surgical procedure of thrombectomy was sufficient as it resulted in restoration of valve function. Delay in treatment is fatal during pregnancy and hence, we should have a high level of suspicion whenever a woman presents with acute heart failure or thromboembolic events with MPHV. TTE is the simplest bedside evaluation that can detect MPHV thrombus. Multidisciplinary care, maternal stabilization, and early institution of heparin/thrombolysis therapy or surgery improve maternal and fetal outcome. Preconceptional counseling, frequent monitoring, measures to increase the compliance to anticoagulants use in pregnancy such as awareness of complications of various anticoagulants and informed choice of anticoagulants use in pregnancy are the various strategies to reduce the TEC associated with MPHV during pregnancy.
Conflict of Interest
None declared.
References
- Pregnancy and cardiac interventions: what are the optimal management options? J Card Surg. 2020;35(07):1589-1596.
- [Google Scholar]
- Follow-up and management of valvular heart disease patients with prosthetic valve: a clinical practice guideline for Indian scenario. Indian J Thorac Cardiovasc Surg. 2019;35(01):3-44.
- [Google Scholar]
- Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC) Circulation. 2015;132(02):132-142.
- [Google Scholar]
- Pregnancy outcomes in women with mechanical prosthetic heart valves: a prospective descriptive population based study using the United Kingdom Obstetric Surveillance System (UKOSS) data collection system. BJOG. 2017;124(09):1411-1419.
- [Google Scholar]
- Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J. 2017;38(19):1509-1516.
- [Google Scholar]
- Maternal and fetal outcomes of anticoagulation in pregnant women with mechanical heart valves. J Am Coll Cardiol. 2017;69(22):2681-2691.
- [Google Scholar]
- 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(05):e35-e71.
- [Google Scholar]
- 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739-2791.
- [Google Scholar]
- Maternal and fetal outcome after cardiac operations during pregnancy: a meta-analysis. Ann Thorac Surg. 2018;106(02):618-626.
- [Google Scholar]
- A case report: mechanical mitral valve thrombosis in pregnancy. Eur Heart J Case Rep. 2019;3(01):ytz024.
- [Google Scholar]








