Translate this page into:
Heart Failure in Women
*Corresponding author: Jyotsna Maddury, Department of Cardiology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India. janaswamyjyotsna@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maddury J. Heart failure in women. Indian J Cardiovasc Dis Women 2022;7:162-74.
Abstract
Heart failure (HF) in women is one of the leading causes in women after coronary artery diseases. There are gender differences at every aspect of HF. These females usually present in older age with significant comorbidities. Even though there are few risk factors common to males and females for the development of HF, diabetes and hypertension are considered more stronger association for the development of HF in females than in males. There are certain sex-specific factors such as abnormal pregnancies and breast cancer therapies in addition to genetic predisposition for the development of cardiomyopathies, especially postpartum cardiomyopathy in female. Usually, females have HF with preserved ejection fraction when compared to men who more frequently have HF due to reduced ejection fraction. Even in the left ventricular remodeling to injury is different in both sexes. The main aim of this review is to bring the sex differences in HF and to stress the need of separate guidelines for females with HF for better outcome.
Keywords
Cardiovascular diseases
Heart failure
Women
INTRODUCTION
The leading cause of death in women now is cardiovascular disease (CVD).[1] Even though there was a reduction in mortality and morbidity of CVD in different populations, still remains high in young females. Multiple factors such as genetic, hormonal, and environmental factors singularly or in combination may explain this gender difference in CVD.[2,3] In addition, females are underrepresented in clinical trials even though many associations’ recommendations say that at least 30% of the female population to be included in the trials. The guidelines drawn from these female less population will be advised for both sexes.[4]
This disparity of gender is not only seen in a whole of CVD but also in all subgroups of CVD like heart failure (HF). As it is known from the previous studies, females present later, with more symptoms, in older age, with less ischemic cause, less aggressively treated, and higher mortality. In addition, the delay in diagnosis is compounded by the higher prevalence of HF with preserved ejection fraction (HFpEF) in females.[5] This gender difference is present in genetic cardiomyopathies and in drug-induced cardiomyopathies for breast cancer. Furthermore, peripartum cardiomyopathy (PPCM) and pregnancy-associated complications such as preeclampsia are exclusive to women adding to long-term cardiovascular morbidity in females. Autoimmune diseases are also more frequent in females.[6]
The possible reasons for this gender difference may be due to the:[7]
Innate biological differences such as gender-related differences in the manifestation of the disease, treatment response, and the natural history of the disease
Unmeasured clinical variables such as confounding factors (e.g., disease severity or comorbidity) and differences in care as well as the difference in the application of clinical guidelines.
In this review, we want to highlight the gender differences in epidemiology, pathophysiology, response to therapies, and prognosis of HF. Along with the common risk factors for HF, special attention was given to specific risk factors in females, such as reproductive and hormonal challenges. At every step, the underrepresentation of females in the clinical and interventional trails and prominent research gaps was also discussed.
EPIDEMIOLOGY
HF is going to be the leading cause of death in both sexes.[8,9] HF constitutes 1–2% of the general population in developed countries with an estimated 64.3 million people suffering now. By 2030, the expected increase in this HF incidence by 46%, out of the 50%, is going to be with HFpEF.
Even though there is a higher lifetime risk of developing coronary heart disease (CAD) in men before 40 years (one in two for men and one in three for women), by the age of 40, both sexes have equal lifetime risks of developing HF with the incidence of one in five. The incidence of new HF increases with age, the increment is faster in females than males (about double from 65 to 85 years in males whereas it is triple in females of the same age). Even though the overall incidence of HF in males is more than that of females in the younger age group, it equalizes by 80 years. Actually, after 80 years of age, the incidence of HF is almost 20% in males, more so in females.[10,11]
According to the Swede-HF registry, HF in women is mostly due to HFpEF (in 55% HFpEF vs. 29% HF with reduced ejection fraction [HFrEF] patients). This higher percentage of women with HFpEF may be due to differences in the age distribution of the population at risk or higher life expectancy.[12] The data from the Cardiovascular Health Study and the Multi-Ethnic Atherosclerosis Study also support that the lifetime risk for HFrEF was higher in men than women (10.6% vs. 5.8%), but the lifetime risk for HFpEF was similar for both sexes.[13]
STAGES IN HF
HF could be acute or chronic, either left-sided or right-sided HF. There are four stages in HF (Stages A, B, C, and D). Stage A is the person who has a high risk of developing HF such as presence or hypertension (HT) or diabetes or CAD. In Stage B, the patient may have either left ventricular (LV) systolic or diastolic dysfunction but clinically does not have HF. Stage C – where the patient has clinical signs and symptoms of HF. In Stage D, the patient has advanced symptoms despite symptoms. Primary prevention can be implemented in Stages A and B to prevent HF development.
ETIOLOGY OF HF IN WOMEN
Common causes of HF are HT, valvular heart disease, diabetes, and coronary artery disease.[14] These are the common causes of HF in women, especially in postmenopausal women. Less common causes include viral myocarditis and cardiomyopathies induced by alcohol/toxins, tachycardia, and stress. Other etiologies specific for women of HF are PPCM, autoimmune disorders, collagen vascular disorder, cardiotoxicity (such as chemotherapy with doxorubicin, trastuzumab, or other toxins), or genetic cardiomyopathies in select populations. HF due to stress-induced cardiomyopathy otherwise also known as takotsubo cardiomyopathy is one of the reversible causes of HF with a good long-term prognosis and is more frequent in postmenopausal women. In this type of cardiomyopathy, the major difference in gender is the type of stress. In females, emotional stress is the cause or precipitating event as physical stress in the case of males[15,16] [Figures 1 and 2].
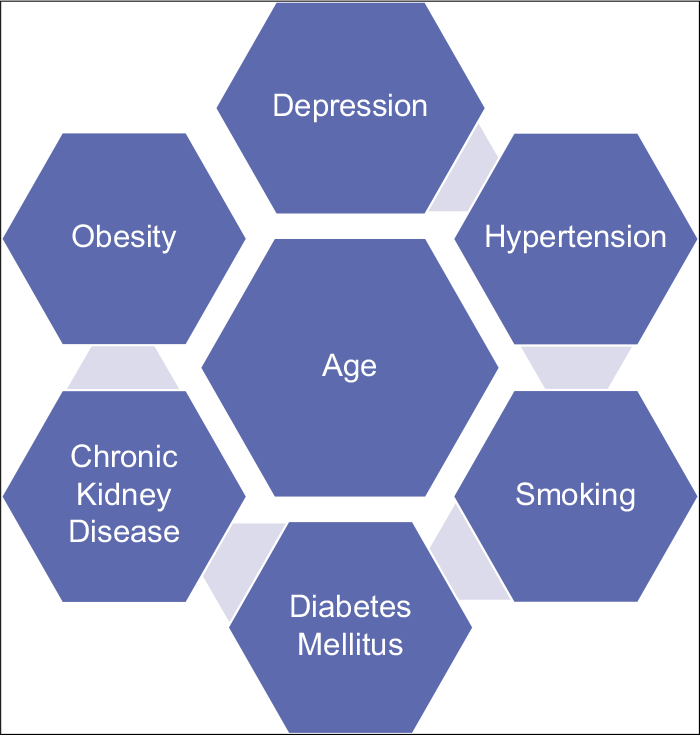
- Traditional risk factors for heart failure development in females.
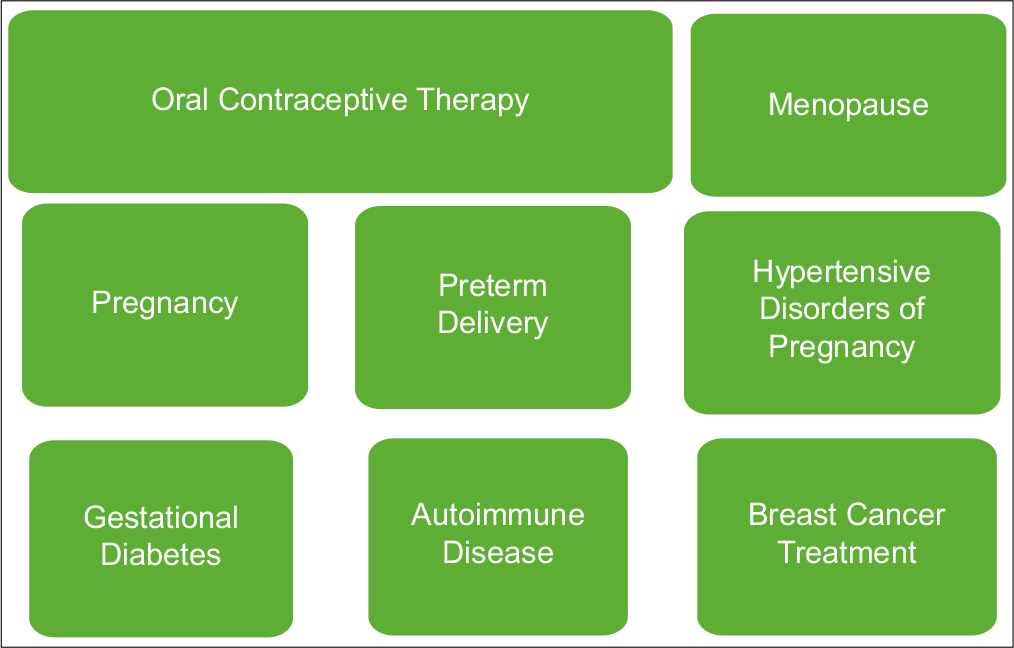
- Non-traditional for heart failure development in females.
SEX DIFFERENCES IN HF
Gender differences are there in every component of HF such as in risk factors, pathophysiology, phenotypes, and outcomes including quality of life [Table 1]. Macrovascular obstruction leads to different varieties of coronary artery disease from myocardial infarction to chronic CAD, leading to decreased LV function causing HFrEF in men. Where in females, the predominant pathophysiology behind HF is the microvascular dysfunction, leading to HFpEF. The same mechanism was implicated even in takotsubo cardiomyopathy and PPCM.[6]
| Women compared to men | |
|---|---|
| Left ventricular mass | Lower |
| Contractility | Greater |
| Cell turnover/apoptosis | Lower |
| Coronary vessel caliber | Smaller |
| Blood pressure | Lower |
| Resting heart rate | Higher |
| Catecholamine-mediated vasoconstriction | Less |
Diabetes mellitus
According to Framingham Heart Study, diabetes was a much more strong risk factor for the development of HF in females than in males (5-fold in women vs. 2-fold in men). Not only that, this study showed that female diabetics have adverse LV remodeling with increased LV wall thickness, relative wall thickness, and LV mass index.[17] Especially Asian diabetic female patients had more concentric LV remodeling with bad long-term prognosis when compared to Asian diabetic men even with low body mass index (BMI), coining the term “lean diabetic phenotype” which predisposes to HF development subsequently. The compounding factors in Asia women are social factors such as low awareness, about the disease and preventive measures along with few cultural practices.[18]
Obesity
Obesity is an important risk factor for the development of HFpEF than HFrEF, more so in women than in men. The prevalence of obesity is more in women thought out the world. For every standard deviation increase in BMI, there is a 34% increase risk of HFpEF.[19] Insulin resistance also increases the risk of HFpEF, which is also more in females than males. Even in normal BMI patients, if they have or develop central obesity (visceral adiposity), which is more prevalent in postmenopausal women, are associated with deterioration of cardiac function. Along with the epidemiologic associations with diabetes and obesity, females are more prone to the risk of developing HFpEF.[20]
HT
HT is an important risk factor for HF, more so in women than men. HFpEF women are more likely to have HT and less likely to have CAD.[21] Framingham’s Heart Study showed that HT has a different effect on the heart differently in men and women. Women with HT develop more concentric LVH versus eccentric LVH than men for a similar increase in LV mass.[22]
Ischemic heart disease (IHD)
Nowadays, there is an increased incidence of IHD in both sexes.[23] However, an important adverse bias is found in women compared to males in terms of both diagnostic and therapeutic measures. Women are less likely to undergo stress testing or coronary angiography and hence less likely to receive preventive treatment. Women receive less revascularization treatment than men ultimately leading to more advanced disease at myocardial infarction, increased incidence of HF, and increased mortality.[24] Special attention should be given to additional mechanisms of ischemia in women such as increased incidence of coronary vasospasm, spontaneous coronary artery dissection, and increased thrombotic burden secondary to autoimmune disorders.
Tobacco smoking
The First National Health and Nutrition Examination Survey (NHANES I) study showed that smoking is a higher risk factor for the development of HF in females than in males (88% in females vs. 45% in males). Even though females smoked “light” cigarettes, they were at risk for HF. On further analysis in the same study, they found that passive smoking was also a strong risk factor for HF in women, may not so much in males. Smoking is an additional risk factor for the PPCM.[25]
Genetics
Genetic cardiomyopathies, that is, hypertrophic and dilated cardiomyopathy, long QT, and Brugada syndrome, are affected by the penetrance, and usually, these cardiomyopathies affect males more severely. Women’s concern point in these genetic cardiomyopathies is that HF can be precipitated during pregnancy.[26,27]
GENDER-SPECIFIC RISK FACTORS FOR HF
Few risk factors are there which are specific only to women. These are reproductive factors, PPCM, and chemotherapy for breast cancer. Even though stress cardiomyopathy can occur in both sexes, it is discussed under this headline as it is more prevalent in postmenopausal women. So also, HF due to inflammatory cardiomyopathies and pulmonary artery hypertension-related right HF due to collagen vascular diseases are also more frequent in women.
REPRODUCTIVE FACTORS
The shorter reproductive period in postmenopausal women is a risk factor for HF. Nulliparous women are more likely to develop HFpEF.[28] Pre-eclampsia, eclampsia, preterm delivery, abruption placenta, and gestational diabetes are known causes for future cardiovascular risk factors which subsequently may lead to HF.[29] According to the Heart and Estrogen/Progestin Replacement Study trial, history of myocardial infarction, active smoking, diabetes, BMI >35 kg/m2, atrial fibrillation, systolic blood pressure >120 mmHg, creatinine clearance <40 mL/min, left bundle branch block (LBBB), and LV hypertrophy were the strongest risk factor for the development of HF.[30]
PPCM
A physiological stress test for women is a pregnancy that can mask the underlying cardiac diseases or predisposes them to new cardiac disease due to its innate effect on endothelial function and inflammatory profile, which, in turn, can lead to HF. PPCM is specific to the female sex and may occur during late pregnancy or in the early postpartum period. In addition to the particular period of pregnancy, the definition of PPCM by the European Society of Cardiology 2010 guidelines includes that there should not be any other known causes of HF with LV ejection fraction (LVEF) should <45% with or without LV dilatation. PPCM is diagnosed and offers many insights into the interplay between sex and HF.[31]
PPCM is a global challenge. The incidence is about one in 3000 deliveries. Even though it is an unpredictable pregnancy-related disease, it occurs more frequently in women >30 years of age, those with a history of pregnancy-associated HT, and in those with multifetal gestations[32] along with genetic predisposition and altered immune status (either the defective immune system or autoimmunity). Contributing factors may be a different type of stress during pregnancy like a hemorrhage of metabolic factors, which may alter the essential cardioprotective signaling pathway.[33] It is better prognosis and it is detected early and treated promptly. About 50% of females recover completely, the rest of the PPCM patients may have partial recovery or downhill course, leading to chronic HF.
BREAST CANCER
It is surprising to know from the epidemiologic studies that therapy-related morbidity and mortality are more than disease-related mortality for CVD in long term in breast cancer surviving women.[34] Anthracyclines and trastuzumab used in breast cancer chemotherapy are associated with a significant decline in LVEF adding to the burden of cardiovascular morbidity and mortality in these women. Because of the side effect of drugs, there is interruption of drug usage, leading to under-treatment of cancer as well as worsening of symptoms of HF due to already occurring side effects, ultimately leading to bad outcomes.[35] Not only chemotherapy even radiotherapy and ovarian suppression therapy have long-term cardiovascular effects, especially HFpEF.[36]
TAKOTSUBO CARDIOMYOPATHY
Takotsubo (stress) cardiomyopathy is an another important sex predilected HF defined as an acute and transient HF condition due to excess sympathetic activity, leading to a ballooning of LV (apical hypokinesia and basal hypercontractility). It is more frequent in females (F:M:9:1).[37] Usually, the stress is the precipitating event that causes excess sympathetic surge and leads to myocardial stunning (adrenoceptor-mediated damage, direct catecholamine toxicity, increased cardiac workload, and epicardial and microvascular coronary vasoconstriction and/ or spasm). Even there is an evidence to say that myocardial stunning is the neurogenic origin as innervated coronary microcirculation, vasoconstrictive in this condition. There is a sex difference in the stress which precipitates this condition. In men usually, it is physical stress, whereas in women, it is mental stress. In fact, it was shown that mental stress-induced myocardial ischemia and a greater increase in platelet reactivity occur in women than in men.[38] Not only that the previous studies proved that psychological distress was proved to associated with CVD events in females, but not in males.
PATHOPHYSIOLOGY OF HF IN WOMEN
Innately, there are significant pathophysiologic differences in the cardiovascular systems of women and men, which can lead to the different manifestations when HF occurs in both sexes.[39]
Compared to men, women have a faster resting heart rate, lower blood pressure, lower LV mass, preserved LV mass with aging, greater LV contractility, less catecholamine-mediated vasoconstriction, small coronary vessels, and a lower rate of apoptosis.
In normal women, LV mass and size are smaller. With aging normally, the LV mass increases in women whereas in men, it decreases. In addition, whenever this is injury or increased workload then disproportionate increase in LV mass occurs in women when compared to men [Figure 3]. With similar degrees of clinical impairment, women have better systolic cardiac performance than men. Hence, women more frequently have HFpEF. This disproportionate increase in LV mass is true for women with aortic stenosis (AS). These women tend to have smaller and thicker LV when compared to men.[39,40]
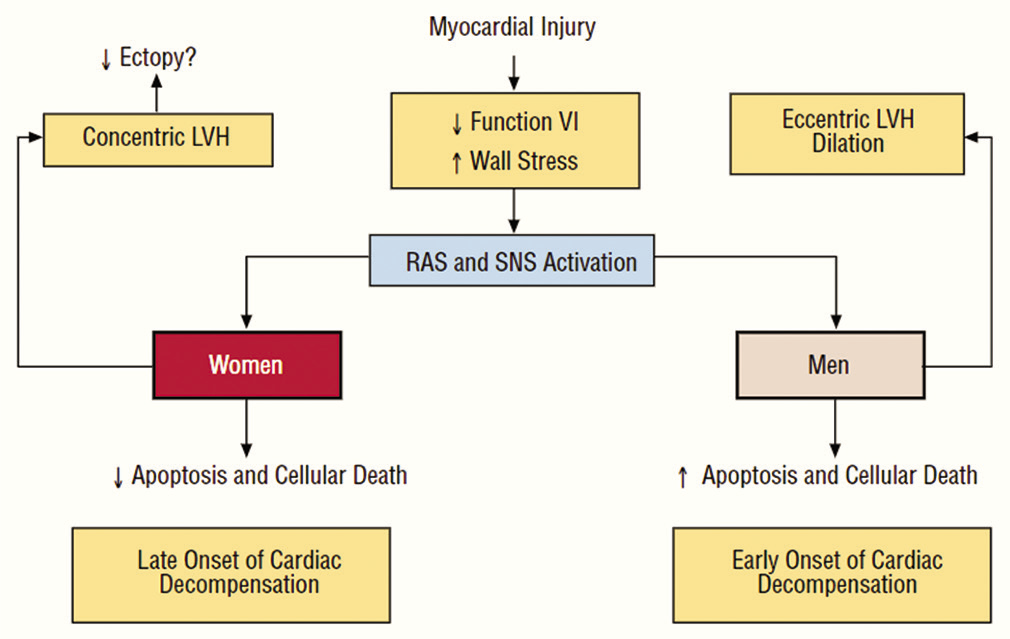
- The difference in the left ventricular hypertrophy as a response to myocardial injury in both sexes.
Some other authors felt that the gender differences in HF may be explained by the sex hormones differences in both genders [Table 2]. Estrogen decreases the renin activity, so vasodilatation with decreased tissue fibrosis, producing the protective effect for HT development, whereas testosterone has the opposite effect.[23] Because of these sex hormone changes men are more prone to macrovascular dysfunction, leading to MI and HF due to LV systolic dysfunction, whereas women are more prone to microvascular dysfunction, leading to HFpEF.[41]
| Site | Androgens | Estrogens |
|---|---|---|
| Heart | ||
| Contractility |  |
 |
| Left ventricular mass |  |
 |
| Fibrosis |  |
 |
| Vessels | Vasoconstriction | Vasodilation |
| Musculoskeletal |  |
 |
| Kidneys | ||
| Glomerulosclerosis |  |
 |
| Renin |  |
 |
Supportive evidences for the endothelial inflammation-coronary microvascular dysfunction in women are
Takotsubo cardiomyopathy – neurogenically triggered coronary microvascular dysfunction
PPCM – oxidative stress-mediated cleavage of prolactin may inducing endothelial damage
Radiotherapy-induced cardiomyopathy – in breast cancer, microvascular endothelial damage occurs after radiotherapy, leading to microvascular rarefaction and myocardial inflammation, which, in turn, leads to more oxidative stress and fibrosis, manifesting as cardiomyopathy[27,37]
Angina with normal coronaries – again microvascular dysfunction was implemented as the cause of angina.
All the above-mentioned diseases are common in women, which give clue for the “common soil hypothesis” to explain when women have HFpEF more frequently.[42]
Even immune response in women high when compared to men may be partially due to the difference in endothelial damage. Women have heightened immune response, leading to greater pro-inflammatory cytokines, more activation of T lymphocytes, and high C-reactive protein levels.[43] This effect is partial because of pro-inflammatory gene expression upregulation in the female myocardium, along with peripheral inflammatory activation. This hypothesis is further substantiated by the fact that autoimmune diseases are also common in women [Figure 4]. In fact, the autoimmune diseases themselves are associated with the development of diastolic dysfunction.[44]
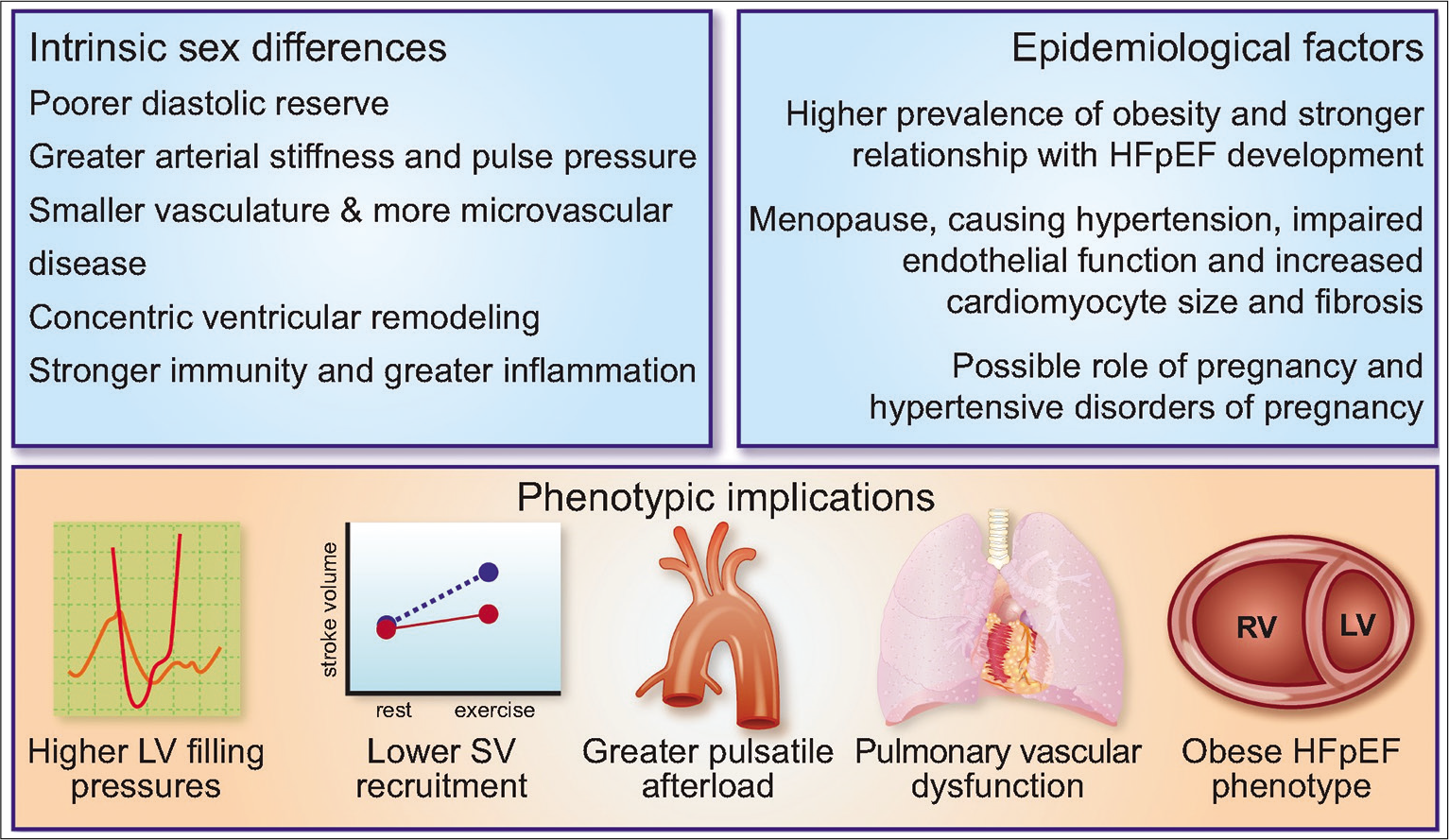
- Factors contributing for gender differences in HF – epidemiological factors, phenotypic implications, and intrinsic sex difference.
Another important cause leading to the HF is, abnormal pregnancies leading to HF on long term follow up. In women, special emphasis should be given to the pregnancy-associated complications such as pre-eclampsia, gestational diabetes mellitus, and placental syndromes. These complications lead to endothelial dysfunction and inflammation which persist in a number of females predisposing these females to increased risk of CVD decades later [Figure 5].
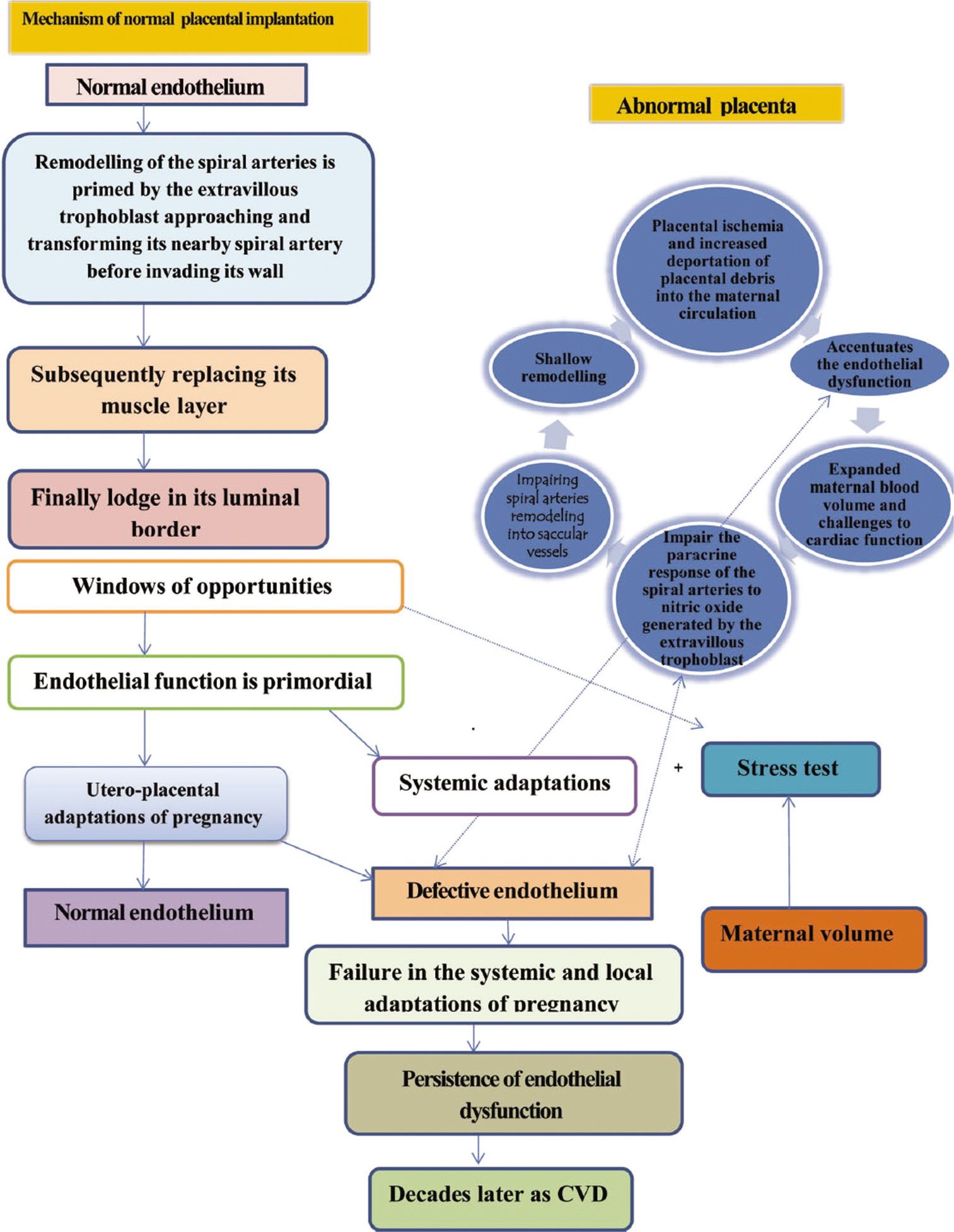
- Pathophysiology of complicated pregnancy, leading to long-term maternal effects.
PRESENTATION OF HF IN WOMEN
HF occurs more in old-age women than men. For similar low LVEF with HF, women are more symptomatic than men.[45] Even after adjusting for age, EF and NYHA class females with HF have the worse quality of life.[46] Women with HF have more frequent pedal edema, exertional dyspnea, and less exercise capacity than men. Depression is more frequent in females.[47] There is no difference in signs of HF in males and females. Gender bias is existing in the present era also for referrals, revascularization, implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT), or mechanical circulatory support.
DIAGNOSIS
Along with clinical signs and symptoms of HF, investigations are also important to diagnose and prognostic of HF such as are NT pro-BNP, and echocardiogram. In some cases, computed tomography scan or magnetic resonance imaging, and coronary angiogram are also important. Although the same tests are used to diagnose HF in women and men, the results may not be the same. The LV dimensions including volumes should be indexed to body surface area (BSA), as these normal values depend on age and sex.[48] Even biomarkers for HF that is natriuretic peptide levels are usually higher in women than in men.[49] Women have lower peak VO2 than men, which may be due to a higher percentage of body fat than men. Hence, indexing to BSA is important.[50]
The poor prognosis of HF in females in addition to natural differences between the genders is a delay in referral, diagnosis, and limited invasive management in females. They often do not receive guideline-directed care. Usage of sex-specific cutoffs for high-sensitivity troponin I may detect more females with MI and early treatment can be started. Compounding factors to angina or MI management are more frequent non-obstructive coronary artery disease (MINOCA). Even though females with MINOCA have fewer chances of having a recurrence of MI, the mortality rate is similar to men. However, this subset of patients does not receive important drugs such as statins and angiotensin-converting enzyme inhibitors (ACEIs), which are proven to have mortality benefits, as there are normal coronaries.
MANAGEMENT
Drug therapy
There is no difference in guideline-directed medical therapies between both genders except in pregnancy. There are a few drugs (ACEI, Angiotensin receptor blockers [ARBs], and neprilysin inhibitors) that should be avoided as there have been suspected teratogenic potential.
There are differential gender responses to pharmacological HF therapies due to sex differences in the pharmacokinetics and pharmacodynamics of the HF drugs. With similar doses, ACEI, ARBs, and beta-blockers can be up to 2.5 times higher in women than in men (may be due to low body weight, higher body fat along with the lower peripheral distribution volume), so adjustment of the dosage to prevent the side effects should be done in female HF patients. Not only there are pharmacokinetic gender differences but there are pharmacodynamic differences also.[51,52] For example, a greater reduction of blood pressure and heart rate occurs in females than in males for similar doses.
Even this gender-based difference in drug therapy for HFrEF also exists. In female patients with HF, when they reach 50–60% of the target dose of beta-blocker and 40–60% of the target dose of ACEI/ARB, there is a 30% reduction in mortality. Further increasing the dose of either beta-blocker or ACEI/ARB is not giving mortality benefit for these female patients, except for an increase in the side effects, whereas for males, reaching the target dose is beneficial. Hence, there is a need to find out the sex-based target levels for these drug therapies.[53] Overall adverse events in females, from HF drug therapy, are twice the rate in men. In the HEAAL trial, a similar effect with a lower dose of losartan was demonstrated in females.[54] Even though the Digitalis Investigation Group trial is a positive trial overall, sub-analysis in females showed higher mortality, because of higher digoxin levels in females for similar doses of males. Now, ongoing digoxin trials are being conducted to see where the lower levels of digoxin are beneficial in females with HF.[55]
In the TOPCAT trial, in patients with HFpEF, spironolactone has not shown improvement over the different EFs in males but was effective over the wide spectrum of EF in females.[56] In the PARAGON-HF trial also sacubitril/valsartan, compared with valsartan showed benefit in females (27% reduction of hospitalization and mortality) with HFpEF but not in males. PARAGON trial showed robust sex interaction even after adjusting other parameters such as EF.[57] The major advantage of the PARAGON trial is that nearly 2479 female patients were recruited, ever largest recruitment of female patients in HF studies.
The proposed mechanisms for these sex differences are sex differences in microvascular inflammation or sex-dependent regulation of the constitutive NOSs or difference in dose-response relationships or greater natriuretic peptide deficiency, which requires confirmation by further studies.
ICD therapy
Even after adjusting for age and compounding factors, women less likely to receive ICDs whenever are indicated. There is doubt about the efficiency of ICD in women in meta-analysis. Moreover, also ICD shocks as well as appropriate anti-tachycardia pacing therapy were fewer in women. The majority of these HFpEF females do not have myocardial ischemia due to major coronary artery disease less likely to have a scar and scar-related ventricular arrhythmias, so ICD may be less efficient, especially in the prevention of sudden cardiac deaths in them.[58] Females have higher rates of device implantation-related complications (bleeding, tamponade lead dislodgement, infection, and pneumothorax). For all these reasons, it is better to individualize each female depending on the risk-benefit profile before ICD implantation.[59]
CRT in women
Contrary to ICD therapy, females respond better than males to CRT, in the form of improvement in quality of life, LVEF, and mortality. This may be due to less obstructive CAD and less myocardial scar as well as the smaller height and heart size.[60] Females require a lower QRS duration cutoff for CRT in the presence of LBBB than men.[61]
Mechanical circulatory devices
Mechanical circulatory devices are useful in both sexes as a bridge-to-transplant or destination therapy. The challenges in getting a donor for females are 2-fold. First, smaller BMI so age-matched donors of smaller size are difficult. Second, multiparous women have significant reactive antibodies, which again preclude the transplantation chances.[62]
Males receive this therapy in 77% of cases according to the International Society for Heart and Lung Transplantation. This registry also reported that mortality rates are higher in females with circulatory assist devices. Even though the continuous flow LV assist devices proved equal efficiency in both sexes, a major limitation is the women with less BSA and small thoracic cavities. For this machine usage minimum, BSA required is >1.5 m2 BSA. Hence, there is an urgent need to design smaller LV assist devices for females.[63]
Cardiac rehabilitation program
Cardiac rehabilitation programs which are an essential component in the management of HF to improve the quality of life, improvement in psychosocial state, and reduce repeat hospitalizations are used less in females. Females are not referred for a rehabilitation program, but when they follow the advice of rehabilitation, and then, females achieve similar or greater benefits than males. Failure to refer may be multifactorial, such as less social support or more burden as caregiver or family responsibilities, older age with more already excising disabilities, and less fitness so not able to take care self so on so forth. Actually, moderate-to-vigorous intensity training and high-intensity interval training are advisable in females with long-term good results. Regular aerobic exercise in sedentary middle-aged women is also proven to be beneficial. In early onset menopausal females, starting moderate endurance training in midlife is helpful. Even in young females with PPCM also rehabilitation programs are helpful. Overall, all women irrespective of age cardiac rehabilitation programs showed benefits and this is indicated.[6]
Management of HF during pregnancy
Special mention is required about the management of HF during pregnancy. Table 3 shows the list of drugs which are contraindicated or to use cautiously were mentioned.[64]
| Drug | Safety during pregnancy |
|---|---|
| ACEI/ARB | Contraindicated because of renal and other fetal toxicity |
| Hydralazine and long-acting nitrates | Safe to use |
| β-blocker | β1 selective drugs preferred, β2-receptor blockers have antitocolytic effect. |
| Diuretics | Should be used sparingly as can cause reduced placental flow |
| Frusemide and hydrochlorothiazide | Can be used |
| Aldosterone antagonists | Teratogenic, to be avoided |
| Amiloride | Can be used, not teratogenic |
| Digoxin | Can be used |
| Ivabradine | No known data, to be avoided |
| ARNI | Contraindicated |
| Amiodarone | Contraindicated, hypothyroidism in 9%, hyperthyroidism, growth retardation, and premature birth |
| UFH and LMWH | Can be used in the prevention and treatment of thromboembolism, mechanical valve, and atrial fibrillation |
| NOACs | Contraindicated |
| VKA | Teratogenic, to be avoided in first trimester, can be used in second trimester if dose of acenocoumarol<3 mg or phenocoumarol<5 mg |
| Nitroglycerine | Can be used in ADHF management secondary to hypertension, left ventricular dysfunction, or severe valve regurgitation |
ACE: Angiotensin-converting enzyme, ARB: Angiotensin receptor blocker, ARNI: ARBs and neprilysin inhibitor, UFH: Unfractionated heparin, LMWH: low-molecular-weight heparin
Even though PPCM is known to occur in the peripartum period, there other conditions such as congenital heart disease or valvular heart disease or pulmonary embolism can produce HF at any stage of pregnancy. In Figure 6, the algorithm to follow in this type of complicated pregnancy is mentioned.[64] In Figure 7, the overall management principals are mentioned for HF complicated pregnancy.

- Steps to follow in women with heart failure during pregnancy.

- Algorithm for the management of acute heart failure during pregnancy.
FEMALE REPRESENTATION IN CLINICAL TRIALS
Even though the clinical trial registry recommended a minimum of 33% of females or depending on the prevalence of the particular disease, to be included in clinical trials, to effectively conclude the solutions, in practice, it does not happen. About <25% of females with HF were included in all the studies[39] except in A-HoFT and Companion trials [Table 4]. There is no decided study on HF in females. As already mentioned due to differences in pharmacokinetic and pharmacodynamic properties of drugs along with small stature, dedicated HF trials only in females are required.
| Trial | % of women represented | Mortality reduction in subgroup analysis |
|---|---|---|
| A-HoFT | 40 | HR 0.33 (0.16–0.71) |
| CIBIS II | 19 | RR 0.52 (0.30–0.89) |
| Comet | 20 | HR 0.97 (0.73–1.27) |
| Companion | 32 | NA |
| Consensus | 20 | RR 1.14 (0.68–1.90) |
| Emphasis-HF | 22 | HR 0.65 (0.4–0.9) |
| EPHESUS | 29 | NA |
| MADIT II | 16 | HR 0.57 (0.28–1.16) |
| MERIT-HF | 23 | RR 0.93 (0.58–1.49) |
| PARADIGM-HF | 21 | HR 0.92 (0.6–1.1) |
| RALES | 27 | NA |
| SCD HeFT | 24 | HR 0.96 (0.58–1.61) ICD arm HR 1.17 (0.72–1.90) Amiodarone |
| Shift | 23 | NA |
| Solved | 20 | RR 1.15 (0.74–1.78) Prevention RR 0.86 (0.67-1.09) Treatment |
| TOPCAT | 51 | HR 0.89 (0.71–1.12) |
HF: Heart failure
PREVENTION OF HF IN WOMEN
As the prevention is better than after the manifestation of HF, different preventive strategies are required for HF with reduced and preserved EF.
The targets and strategies to prevent HF with reduced EF with ischemia are blood pressure control, reaching target lipid levels, cessation of smoking, exercise, and preservation of the myocardium, whereas the targets are different in HFrEF without ischemia. The preventive measures of HF in this condition are paying attention to adjusting the appropriate dose of anticancer therapy, complicated pregnancies management to do in a tertiary care center with a multidisciplinary approach, reduction of both mental and physical stress to prevent stress-induced cardiomyopathy, and measures to prevent the microvascular disease and effective management of diabetes.
The targets and strategies to prevent HF with preserved EF are weight reduction, blood pressure control, preventive measures for atrial fibrillation, exercise, control of inflammation, preservation of endothelial function, prevention of myocardial fibrosis, insulin resistance, and microvascular diseases.
Depending on the stage of HF, either primary or secondary prevention strategies can be planned [Table 5].[65]
| Primary |  |
Secondary |  |
|---|---|---|---|
| Stage A | Stage B | Stage C | Stage D |
| HfrEF | |||
| CAD risk factor management Exercise/Physical activity | Treat HTN, HLD, DM2 Exercise/physical activity Smoking cessation ACE/ARB and BB |
ACE/ARB and BB Sodium restriction Diuretics Mineralocorticoid-receptor antagonist Neprilysin inhibition Cardiac rehabilitation |
Cardiac resynchronization therapy Inotropes Left ventricular assist device Heart transplant |
| HfpEF | |||
| Maintain a healthy weight Weight loss for overweight and obese women Statin Blood pressure control Exercise/Physical activity | Treat HTN Weight loss for overweight and obese women Glucose control in women with DM2, pre-diabetes or history of GDM Physical activity |
Treat HTN Diuretics Sodium restriction Use of new antiglycemic agents Neprilysin inhibition |
Treat HTN Diuretics Supportive care |
HTN: Hypertension, GDM: Gestational diabetes mellitus, CAD: Coronary heart disease, HF: Heart failure, ACE: Angiotensin-converting enzyme, ARB: Angiotensin receptor blocker, HfpEF: HF with preserved ejection fraction, HFrEF: HF with reduced ejection fraction
PROGNOSIS
In the past five decades, the survival rates are improved in both sexes, even though there is increase of HF more in women than in men.[66] There are larger survival gains in men and young HF patients than in women and the elderly. Women with HF, on the whole, have better survival than men, especially this difference is more prominent when HF is of non-ischemic origin. In ischemic cardiomyopathies, there are no sex differences in mortality, over that females twice likely to develop HF after MI than men (which may be partially due to under-treatment), even though females have better EF.[67]
Similar sex difference exists in HFpEF also. Even females have more severe HF clinical symptoms, there is no increase in the rate of hospitalization due to recurrence of HF. In fact, females with HFpEF have better survival than men.[68]
PROPOSED RECOMMENDATIONS FOR FEMALES WITH HF
Even though the risk factors profiles and clinical symptomatic status are more in females, they are underdiagnosed, undertreated, and underrepresented in clinical trials.
Important physiological stress in female is pregnancy, which is an important risk factor for HF development and management differs.
Few of cancer therapies unique to females.
Major pathophysiology is that the HF in females is nonischemic microvascular dysfunction with HFpEF.
Individualize in each female depending on risk–benefit profile before ICD implantation.
There is an urgent need to design smaller LV assist devices for females.
Overall, all women irrespective of age cardiac rehabilitation programs showed benefit and this is indicated.
Pregnant women with HF require multidisciplinary approach.
Dedicated HF trails and guidelines only in females with HF are required.
CONCLUSIONS AND FUTURE DIRECTIONS
There is a gender difference at every component of HF, from epidemiology to pathophysiology to adoption of LV to the management. Diabetes and HT are stronger risk factors in females when compared to men. HFrEF is more frequent in males, whereas HFpEF in females. Mental stress is cause for takotsubo cardiomyopathy in females, whereas physical stress in the precipitating factors in males. There are some sex-specific risk features only to females such as pregnancy-related HF as well as breast cancer-related therapy side effects.
There are large gaps in the knowledge of HF in females, as there are underrepresented trails and guidelines are formed from the male predominant studies, for similar dose, the drug response is worse in females. The major knowledge gaps in females with HF are lack of understanding and appreciation of sex-specific risk factors, limited understanding of pathophysiology of HF especially in takotsubo cardiomyopathy, lower quality of life, higher rates of medication-related adverse events, greater adverse LV remodeling, no female specific criteria for device implantation and more importantly lack of data and guidelines on optimal management of medication, and dosage for females. Hence, there is an urgent need of separate clinical trails only in females and formulate the appropriate guidelines for HF management in females.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 2020;141:e139-596.
- [CrossRef] [Google Scholar]
- Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198-205.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3:163-82.
- [CrossRef] [PubMed] [Google Scholar]
- Enrollment of women in national heart, lung, and blood institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008;52:672-3.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation. 2019;139:1776-85.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in heart failure. Eur Heart J. 2019;40:3859-68.c.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences: Implications for heart failure care. Eur Heart J. 2004;25:101-3.
- [CrossRef] [PubMed] [Google Scholar]
- Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146-603.
- [CrossRef] [Google Scholar]
- Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:E38-60.
- [Google Scholar]
- Lifetime risk for developing congestive heart failure: The Framingham heart study. Circulation. 2002;106:3068-72.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and hospital death rates associated with heart failure: A community-wide perspective. Am J Med. 2005;118:728-34.
- [CrossRef] [PubMed] [Google Scholar]
- The Swedish heart failure registry: A living, ongoing quality assurance and research in heart failure. Ups J Med Sci. 2019;124:65-9.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871-81.
- [CrossRef] [PubMed] [Google Scholar]
- Heart disease in women: A narrative review. Anaesthesia. 2021;76(Suppl 4):118-30.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996-1002.
- [CrossRef] [PubMed] [Google Scholar]
- Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472-9.
- [CrossRef] [PubMed] [Google Scholar]
- Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68:85-9.
- [CrossRef] [Google Scholar]
- Impact of diabetes and sex in heart failure with reduced ejection fraction patients from the ASIAN-HF registry. Eur J Heart Fail. 2019;21:297-307.
- [CrossRef] [PubMed] [Google Scholar]
- The Association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701-9.
- [CrossRef] [PubMed] [Google Scholar]
- A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodelling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263-71.
- [CrossRef] [PubMed] [Google Scholar]
- The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557-62.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310-3.
- [CrossRef] [Google Scholar]
- Heart failure in women. Med Clin North Am. 2004;88:1321-45, xii
- [CrossRef] [PubMed] [Google Scholar]
- Angina and cardiac care: Are there gender differences, and if so, why? Circulation. 2006;113:467-9.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366.
- [CrossRef] [PubMed] [Google Scholar]
- Bioinformatics analysis of sex differences in arrhythmogenic right ventricular cardiomyopathy. Mol Med Rep. 2019;19:2238-44.
- [CrossRef] [Google Scholar]
- Sex-related differences in cardiomyopathies. Int J Cardiol. 2019;286:239-43.
- [CrossRef] [PubMed] [Google Scholar]
- Reproductive factors and incidence of heart failure hospitalization in the women's health initiative. J Am Coll Cardiol. 2017;69:2517-26.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. 2022;130:652-72.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of heart failure among women with coronary disease. Circulation. 2004;110:1424-30.
- [CrossRef] [PubMed] [Google Scholar]
- Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the heart failure association of the European society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767-78.
- [CrossRef] [PubMed] [Google Scholar]
- EURObservational research programme: A worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the heart failure association of the European society of cardiology working group on PPCM. Eur J Heart Fail. 2014;16:583-91.
- [CrossRef] [PubMed] [Google Scholar]
- A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589-600.
- [CrossRef] [PubMed] [Google Scholar]
- A Population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2:88-93.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504-12.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087-106.
- [CrossRef] [Google Scholar]
- Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: Insights from the REMIT study. J Am Coll Cardiol. 2014;64:1669-78.
- [CrossRef] [PubMed] [Google Scholar]
- Heart failure in women. Methodist Debakey Cardiovasc J. 2017;13:216-23.
- [CrossRef] [PubMed] [Google Scholar]
- Heart failure. Are women different? Cardiovasc Dis Women. 2006;59:725-35.
- [CrossRef] [Google Scholar]
- Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439-50.
- [CrossRef] [PubMed] [Google Scholar]
- The parallel tales of microvascular angina and heart failure with preserved ejection fraction: A paradigm shift. Eur Heart J. 2017;38:473-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in immune responses. Nat Rev Immunol. 2016;16:626-38.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-based differences in myocardial gene expression in recently deceased organ donors with no prior cardiovascular disease. PLoS One. 2017;12:e0183874.
- [CrossRef] [PubMed] [Google Scholar]
- Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217-23.
- [CrossRef] [Google Scholar]
- Quality of life in patients with heart failure: Do gender differences exist? Heart Lung. 2001;30:105-16.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma brain natriuretic peptide concentration: Impact of age and gender. J Am Coll Cardiol. 2002;40:976-82.
- [CrossRef] [Google Scholar]
- Comparison of peak exercise oxygen uptake in men versus women in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;80:85-8.
- [CrossRef] [Google Scholar]
- Gender based dosing of metoprolol in the elderly using population pharmacokinetic modeling and simulations. Int J Clin Pharmacol Toxicol. 2016;5:209-15.
- [CrossRef] [Google Scholar]
- Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143-57.
- [CrossRef] [PubMed] [Google Scholar]
- Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur Heart J. 2005;26:1585-95.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): A randomised, double-blind trial. Lancet. 2009;374:1840-8.
- [CrossRef] [Google Scholar]
- Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5:425-38.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402-14.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609-20.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in advanced heart failure therapies. Circulation. 2019;139:1080-93.
- [CrossRef] [PubMed] [Google Scholar]
- Gender and outcomes after primary prevention implantable cardioverter-defibrillator implantation: Findings from the national cardiovascular data registry (NCDR) Am Heart J. 2015;170:330-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-specific difference in outcome after cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2019;20:504-11.
- [CrossRef] [PubMed] [Google Scholar]
- The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: An individual-patient data meta-analysis. Eur J Heart Fail. 2018;20:780-91.
- [CrossRef] [PubMed] [Google Scholar]
- Registry of the international society for heart and lung transplantation: Twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant. 2009;28:1007-22.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515-22.
- [CrossRef] [PubMed] [Google Scholar]
- Heart failure in pregnancy. Indian J Cardiovasc Dis Women WINCARS. 2018;3:161-5.
- [CrossRef] [Google Scholar]
- Primary prevention of heart failure in women. JACC Heart Fail. 2019;7:181-91.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397-402.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol. 1996;28:1781-8.
- [CrossRef] [Google Scholar]
- Comparison of morbidity in women versus men with heart failure and preserved ejection fraction. Am J Cardiol. 2006;97:1228-31.
- [CrossRef] [PubMed] [Google Scholar]







