Translate this page into:
Extracorporeal Membrane Oxygenation Assisted Cardiac Interventions
*Corresponding author: Vivek Gupta, Department of Cardiac Anaesthesia and Intensive Care, Hero DMC Heart Institute, Ludhiana, Punjab, India. dr_vivekg@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta V, Ramanathan KR. Extracorporeal Membrane Oxygenation Assisted Cardiac Interventions. Indian J Cardiovasc Dis Women. 2024;9:176-84. doi: 10.25259/IJCDW_48_2024
Abstract
The use of extracorporeal membrane oxygenation (ECMO) for management of critically ill patients has significantly increased in the recent past due to technological progress and increased experience with safe ECMO runs. The use of venoarterial (VA) ECMO during high risk interventional cardiology procedures ensure adequate cardiac output and other organ perfusion as well. The use of elective VA ECMO support during procedure allow interventionists to perform procedure safely and comfortably, moreover the support may be extended during post-procedural period in case of cardiogenic shock or arrhythmia. VA ECMO may also be instituted in emergent situations when conventional cardiopulmonary resuscitation (CPR) is ineffective to achieve return of spontaneous circulation. The various procedures with high risk potential due to anatomical complexity, haemodynamic decompensation or preexisting clinical condition may require mechanical circulatory support. These include percutaneous coronary interventions, Transcatheter aortic valve implantation (TAVI), complex congenital anamoly or electrophysiological procedures. However an integrated team approach with appropriate communication is vital among interventional cardiologist, ECMO specialist, cardiac surgeon, cardiac anaesthesiologist and perfusionist for a successful outcome.
Keywords
Extracorporeal membrane oxygenation
Percutaneous coronary interventions
Cardiac interventions
INTRODUCTION
The recent past has witnessed an advancement and progressive miniaturization of medical technology in general and cardiac catheterization and transcatheter technology in particular. This has led to rapid increase in complex catheterization laboratory procedures with the challenging anatomical issues and fragile hemodynamics. The hemodynamic fluctuations and arrhythmia in the periprocedural period may compromise the patient’s safety and warrant termination of the procedure or conversion to surgical intervention. However, an integrated approach of interventional cardiologists and extracorporeal membrane oxygenation (ECMO) teams may facilitate performing these complicated interventions safely with ECMO support. Moreover, these catheterization laboratory procedures may be equally efficacious as cardiac surgical interventions and minimize the risk of surgical complications. There is remarkable improvement in outcome in ECMO-supported catheterization in the past three decades.[1,2] The various high risk and complicated procedures potentially needing ECMO support include percutaneous coronary interventions (PCI), transcatheter aortic valve implantation (TAVI), mitral valve interventions, and interventions for congenital anomalies or refractory life-threatening ventricular arrhythmias requiring electrophysiological intervention. Cardiogenic shock is a clinical syndrome with varying degrees of severity ranging from patients at high risk for developing cardiogenic shock (CS) to severe multiorgan dysfunction due to tissue hypoperfusion.[3] Profound CS and refractory cardiac arrest (CA) are associated with poor chance of survival due to extreme circulatory compromise. In both these clinical scenarios, Venoarterial (VA) ECMO provides cardiorespiratory support and sustains the hemodynamics, maintains the circulation, and, thus, improves the tissue perfusion[4] including other organ perfusion as well thus allow interventionists to perform complex catheterization laboratory procedures[5] with protected hemodynamics similar to cardiac surgical operation with cardiopulmonary bypass. This can be initiated and in peripheral vessels (most commonly femoral artery and femoral vein) with quick percutaneous technique in emergent situations. VA ECMO may be initiated as prophylactic support before catheter-directed high-risk procedures or emergently with on-going cardiopulmonary resuscitation (CPR) as ECMO CPR. However, initiating VA ECMO in an emergent situation increases mortality and morbidity risk[6] associated with vascular complications, reduced myocardial reserve due to underlying clinical condition such as severe aortic stenosis (AS) or complex coronary artery disease (CAD) with severe ventricular dysfunction. Prolonged hemodynamic instability in these situations may progress to cardiometabolic shock.[7] Hence, VA ECMO is now considered as safety tool for patient and interventionist during the catheterization laboratory procedures rather than rescue therapy after collapse of the patient with ongoing CPR.[8] The various procedures that may be performed with VA ECMO support include, PCI,[9] TAVI,[10] mitral valve,[11] and electrophysiological procedures for ventricular arrhythmias ablation.[12]
PROPHYLACTIC ECMO
ECMO for protected high-risk PCI
PCI has been accepted as a less invasive alternative to surgical intervention for CAD management in selected patients.[13-15] This expertise is not restricted to routine cases but also is performed in patients with complex and high-risk category.[16] Most of the definition of “high-risk category” include clinical, anatomic, and hemodynamic parameters; however, uniform consensus definition is still lacking. Clinical parameters include cardiogenic shock occurring at PCI initiation or within the first 24 h of the procedure, reduced EF ≤30%, Killip class II–IV, post-CPR survivor requiring PCI within 24 h, hemodynamic instability, or ventricular arrhythmias following acute coronary syndrome. Anatomically unsupported left main disease or left main equivalent, severe multi-vessel CAD, chronic total occlusions, severely calcified lesions, or PCI of a vessel or graft supplying significant area are considered high risk. Hemodynamic parameters include hypotension systolic blood pressure <90 mmHg or need of vasoactive drugs, cardiac index <2.2 L/min/m2, and pulmonary capillary wedge pressure above 15 mmHg with organ hypoperfusion.[17-21]
These cases not only require technical expertise but also require an excellent understanding of lesions and its impact on hemodynamics. Besides, this several other factors such as comorbidities, degree of left ventricular dysfunction, and anticipation of hemodynamic compromise may warrant the need for prophylactic mechanical circulatory support (MCS). Interventional cardiologists traditionally uses intraaortic balloon pump (IABP) or other MCS devices such as Impella or Tandem-Heart in the catheterization laboratory percutaneously. However, these devices have its own limitations including only partial hemodynamic support without providing oxygenation [Table 1]. The selection of appropriate MCS necessitates multidisciplinary decision-making to minimize periprocedural risk.[22,23] VA ECMO with its unique ability to provide respiratory support along with sustaining circulation during high-risk PCI expands the safety window and avoids untoward event throughout the procedure.[24] VA ECMO may offer adaptable and complete hemodynamic support (above 4.5 L/min) even during arrhythmia, improves tissue perfusion, and supports other organ perfusion as well thus enhancing the safety and feasibility of procedures. Hence, VA ECMO support extends much beyond mechanical support during procedure and supporting the hemodynamics after the intervention as well as overall outcome.[21]
| VA ECMO | Impella® | |
|---|---|---|
| Flow mechanism | Centrifugal flow | Axial flow |
| Access and return of blood | Femoral vein to femoral artery | LV to Aorta |
| Flow | Up to 7 LPM | 2.5–5.5 LPM |
| Cannula size | • Access: 23–25 Fr • Return: 15–19 Fr |
13–21 Fr |
| Advantage | • Biventricular support • Maintains oxygenation |
• LV unloading • Easy cannulation • Physiological flow |
| Disadvantage | • Increasing afterload due to retrograde flow • Bleeding and vascular complications |
• Cannot be used in prosthetic aortic valve or LV thrombus |
VA ECMO: Venoarterial extracorporeal membrane oxygenation, LV: Left ventricle, LPM: Liter per minute
The successful clinical outcomes in ECMO supported high-risk PCI are not merely successful hemodynamic support during procedure but include post-procedural hemodynamic support avoiding major adverse cardiac events, myocardial function recovery, and discharge follow-up to evaluate immediate-, short-term, and long-term outcome.[24] However, a careful selection of patient and close monitoring to address ECMO-related complication early has been emphasized during high-risk PCI for successful long-term outcome [25/18/28]. The feasibility of ECMO supported PCI has been evaluated in patients whom surgical revascularization may be high risk due to several factors including co morbidities, age, or poor myocardial function. Current evidence supports the potential use of VA ECMO in these high-risk patients for successful revascularization in catheterization laboratory minimizing the complication and improving quality of life.[25] Although these evidences are encouraging but have several limitations such as retrospective nature of collected data increasing the bias risk, single-center, and small numbers of cases needing more conclusive research.
TRANSCATHETER AORTIC VALVE IMPLANTATION (TAVI)
TAVI is performed routinely for symptomatic severe calcific AS and selected cases of severe aortic regurgitation (AR) with high surgical risk.[26] Even low-risk surgical patients are chosen for TAVI.[27] Several technical factors such as bulky, asymmetric leaflet and calcification create challenges for this procedure. Moreover, TAVI in severe AS with acute cardiogenic shock is associated with poor outcome.[28] Several intraprocedure events may also lead to hemodynamic instability leading to persistent low cardiac output and cardiogenic shock. This includes spontaneous or post-pacing ventricular arrhythmia, poor coronary artery perfusion secondary to hypotension or coronary ostial obstruction due to prosthesis, and bradyarrhythmia due to prosthesis impingement following valve implantation. Patients having severe myocardial dysfunction with pulmonary hypertension deteriorate rapidly while pacing during balloon dilatation or prosthesis deployment. If the medical management fails to optimize the hemodynamics, MCS may be required till recovery.[29,30] Prophylactic VA ECMO support has been used successfully to sustain adequate hemodynamics in specific high-risk group patients.[10,31] However, an appropriate criterion for elective use of VA ECMO during high-risk TAVI procedure is yet to be established.
Electrophysiological procedures
Patients having electrical storm due to malignant ventricular arrhythmia resistant to medical management may radiofrequency ablation, a non-pharmacological approach.[32,33] These patients often have several comorbidities along with underlying CAD or chronic heart failure. These patients are prone for decompensation during procedure which may be more evident during induction of ventricular arrhythmia for mapping.[34] Prolonged hemodynamic instability may progress to cardiogenic shock and multiorgan dysfunction with increased mortality risk. The use of IABP during the procedure has not shown any survival benefit.[35] However, the use of prophylactic VA ECMO permits adequate circulation during prolonged ventricular arrhythmia during procedure and reduces the risk of potential complications, thus improving the outcome.[36,37] However, careful ECMO management is essential to reduce the ECMO-related complications.
Cardiac catheterization in pediatrics
Elective VA ECMO placement in high-risk pediatric and neonatal cardiac catheterization procedures has shown a favorable outcome as compared to placement of ECMO with on- going CPR.[38]
PREPARATION PHASE BEFORE PROCEDURE
A multidisciplinary team including cardiologists, cardiovascular surgeon, cardiac anesthesiologists, ECMO specialist, and perfusionists must discuss the clinical situation, possible complication risk during procedure and need of prophylactic extracorporeal support. This situation may arise when cardiac surgical option is precluded due to high operative risk or catheterization laboratory intervention is planned in high-risk patients. Euroscore II, Syntax score, etc., may be a good assessment guide in addition to a thorough clinical, anatomical, and hemodynamic assessment. All these factors must be taken in consideration for mechanical circulatory assistance before performing percutaneous coronary or valve procedures.[39] Hemodynamic instability needing vasoactive drugs to maintain arterial blood pressure or electrical instability in the past 24 h with presence or relapses of ventricular arrhythmias may warrant the need of prophylactic VA ECMO before performing the procedure to ensure adequate perfusion and safety.
ECMO MANAGEMENT
ECMO cannulation and initiation
The standard peripheral VA ECMO approach with femoral artery and femoral vein cannulation is appropriate during catheterization laboratory procedure similar to placing VA ECMO in intensive care unit (ICU). However, in cases with difficult arterial access such as extreme atheroma load, reduced vessel caliber or bilateral femoral arterial graft axillary artery may be used for cannulation surgically as an alternative. The usual cannulas size arterial (17–19 Fr) and venous (25–27 Fr) is used for adult patients. The placement of cannula may be percutaneous under ultrasound guidance, fluoro guidance in catheterization laboratory or may be placed surgically under direct vision. A distal perfusion cannula should be placed to ensure adequate perfusion to prevent distal limb ischemia.[40] ECMO circuit consists of venous and arterial lines connected to a centrifugal pump, oxygenator, and heat exchanger, along with pressure monitoring lines to assess the efficacy of oxygenator [Figure 1]. The VA ECMO settings are adjusted according to adequacy of perfusion and hemodynamics, patient’s pathology, or technical issues related with procedure. An additional need of left ventricular unloading may arise in certain cases such as severe AR requiring TAVI. The left ventricular (LV) unloading during VA ECMO prevents rise in the myocardial oxygen demand and ischemia minimizing the risk of hemodynamic instability and arrhythmia. One of the nonsurgical options may be chosen by introducing a catheter in the left ventricle (at least 6 Fr) through transseptal or transaortic approach.[41,42] Moreover, an additional port on the arterial line can be added to perform PCI or TAVI procedures with same arterial access. However, a careful monitoring of flow ensures systemic perfusion and monitoring the change in post oxygenator pressure minimizes the risk of hemolysis.
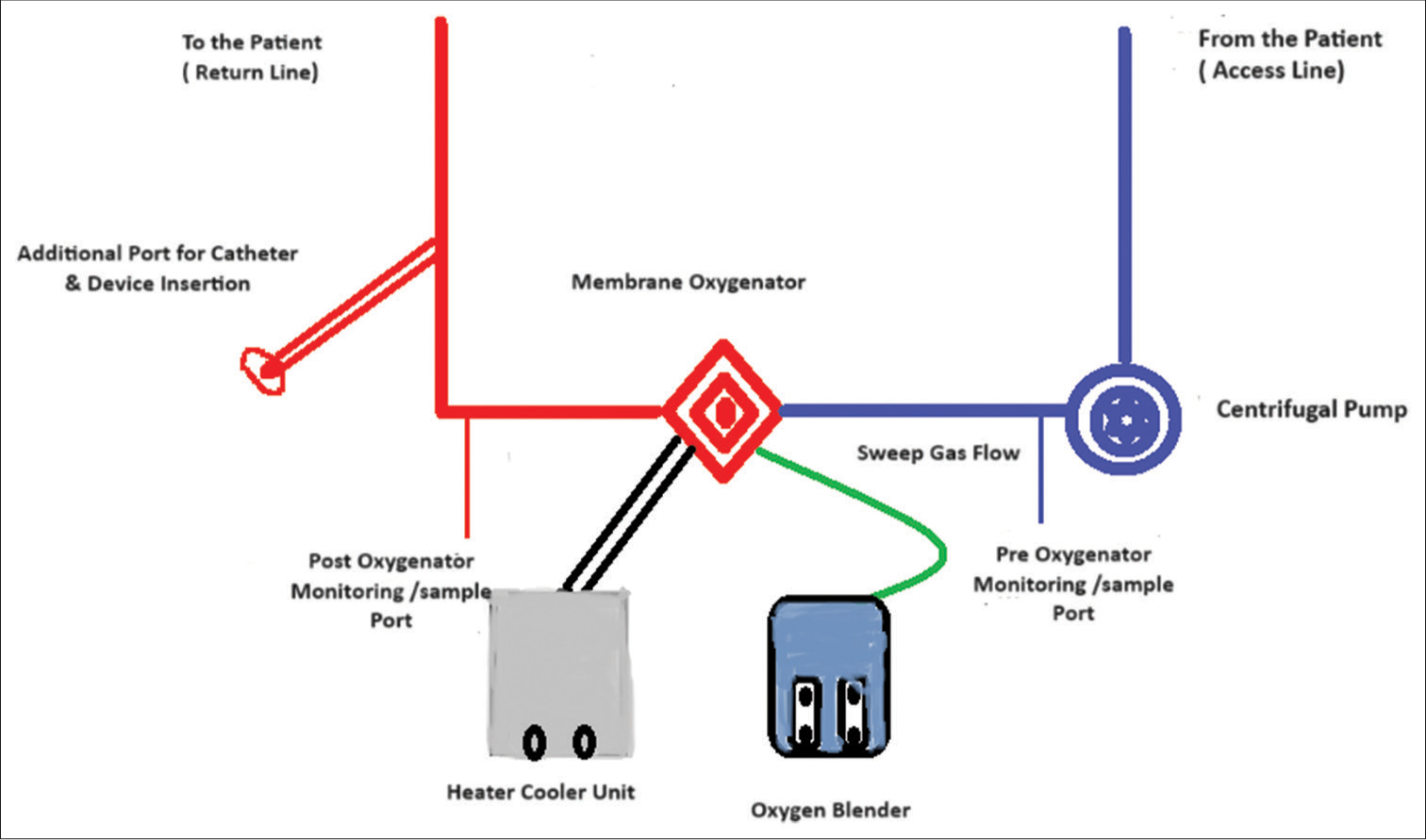
- Venoarterial extracorporeal membrane oxygenation configuration with an additional port for procedure.
In certain cases such as the tricuspid valve intervention, a separate venous cannulation using femoral and internal jugular vein is required to ensure adequate access to tricuspid valve.
Monitoring and management during procedure
The placement of VA ECMO and planned procedures may be performed under local anesthesia and conscious sedation. However, general anesthesia with intubation and mechanical ventilation is suitable if transesophageal echocardiography evaluation is required during the procedure. During general anesthesia, mechanical ventilation and ECMO sweep gas flow is adjusted to avoid hyperoxia and achieve normocarbia. Mechanical ventilation settings can be set to generate minimal tidal volumes during ECMO. Continuous invasive arterial blood pressure monitoring should be initiated along with securing central venous access, which can be helpful for administering vasoactive drug and rapid fluid administration in emergent situations. The use of ultrasound helps in identifying vessel caliber and confirming appropriate position of canula. Heparin bolus is administered as anticoagulation while placing the canula and activated clotting time (ACT) is monitored regularly with a target around 250 sec during the procedure. The heparin infusion should be started to maintain the ACT target. Arterial blood gas, lactate levels, and urine output should be monitored to ensure metabolic, respiratory perfusion adequacy. In most of the cases, the required ECMO flow is below 2/3rd of a patient’s cardiac output, calculated using body surface area. A close communication among interventionist, cardiac anesthesiologist, and perfusion team is crucial to avoid any untoward event during procedure.
The ECMO flow adjustment may be required to meet hemodynamic and metabolic parameters as well as for safe and appropriate execution of procedure. For example, during aortic valve placement, a reduction in flow reduces the chance of pushing the device towards the left ventricle, whereas atheroablative manoeuver during PCI requires an increase in ECMO flow to avoid hypoperfusion and intracoronary stasis of the debris.
POST PROCEDURE: ECMO WEANING AND DECANNULATION
Weaning from ECMO and decannulation may be initiated immediately after an uneventful procedure. However, pre-procedural courses, other organ function and adequate tissue perfusion must be taken in consideration before weaning decisions. Vasoactive drugs infusion may be required to maintain the hemodynamics during weaning. A close and continuous hemodynamic monitoring along with echocardiographic and metabolic assessment is essential during weaning process. Usually, it may not take too long but in cases, where weaning is expected to exceed an hour, patient may be shifted to ICU for further weaning and decannulation. The standard method of VA ECMO weaning is followed in these cases by reducing ECMO flows, before decannulation. The usual method is surgical decannulation with repair of arterial cannulation site; however, sealing hemostasis devices are getting popular for percutaneous closure of cannulation sites, while firm pressure followed by pressure dressing is sufficient for venous cannulation site.
COMPLICATIONS AND CHALLENGES ASSOCIATED WITH ECMO
ECMO associated bleeding especially with VA ECMO is most common complication.[43] Bleeding may occur at cannulation site especially with surgical cannulation; however, bleeding on other sites such as gastrointestinal tract, retroperitoneal area, thoracic, or abdominal cavity may also occur possibly due to systemic anticoagulation, hemodilution, platelet dysfunction, and ECMO-associated systemic inflammatory response.[44] Monitoring-activated partial thromboplastin or other viscoelastic test help in achieving appropriate balance between bleeding and risk of thrombosis during ECMO.[45]
Vascular complications during cannulation not only increase the bleeding risk but may complicate with hemodynamic instability and even failure of ECMO initiation.[46] These vascular complications include arterial dissection, pseudoaneurysm, retroperitoneal bleeding, and increased risk of infection.[47]
Other possible complications specific to femorofemoral VA ECMO is upper body differential hypoxia due to residual myocardial function with severe pulmonary dysfunction may leading to coronary and cerebral ischemia.[48] The differential hypoxemia popularly described as Harlequin syndrome characterized by upper body hypoxemia due to recovering myocardium with poor lung function which may lead to cerebral or coronary ischemia. This requires continuous monitoring oxygen saturation through right hand or arterial blood gas sampling from the right arm along with frequent neurological assessment. The measures to improve upper body oxygenation include increasing ventilator oxygen, positive end expiratory pressure, and other maneuvers to improve gas exchange. However, in extreme situations, venoareteriovenous ECMO or central ECMO may be required. VA ECMO increases the afterload which may lead to left ventricular distension due to restricted aortic valve opening. Medical management using inotropic support may be sufficient in mild LV distension or IABP insertion may help in reducing afterload. In severe LV distension atrial septostomy, surgical venting or Impella® may be helpful. Acute limb ischemia on arterial cannulation side is a devastating complication which may progress to compartment syndrome needing amputation due to inadequate distal blood flow.[49] Distal perfusion cannula placement may reduce the risk of limb ischemia; however, a regular monitoring of limb perfusion is essential for early detection. The long-term outcome of ECMO-assisted procedures has been evaluated and has shown a better survival.[50]
REFRACTORY CARDIAC ARREST AND APPROACH TO E CPR IN CATHETERIZATION LABORATORY
CA during catheterization laboratory intervention creates a significant challenge due to difficulty in continuing intervention in coordination with on-going CPR. A common scenario is patients with ST elevation myocardial infarction (MI) with cardiogenic shock undergoing primary PCI throwing arrhythmias periprocedurally.[51] The E CPR outcome in terms of survival as well as neurological recovery in the female population is better as compared to male population.[52] Even patients undergoing high-risk PCI and complex structural interventions with other comorbidities and poor cardiorespiratory reserve are at high risk for CA during procedure [Table 2]. In cases of CA, a high quality CPR and early institution of VA ECMO with on-going CPR may help in sustaining blood flow and allow the on-going intervention [Figure 2]. A pre-primed ECMO circuit in this emergent situation may facilitate an early ECMO initiation and reducing low flow time[53] [Table 3].
|
MI: Myocardial infarction, PCI: Percutaneous coronary interventions, TAVI: Transcatheter aortic valve implantation, CPR: Cardiopulmonary resuscitation, SCAI: Society for cardiovascular angiography and interventions
|
MSC: Mechanical circulatory support, ECMO: Extracorporeal membrane oxygenation, CA: Cardiac arrest, CPR: Cardiopulmonary Resuscitation
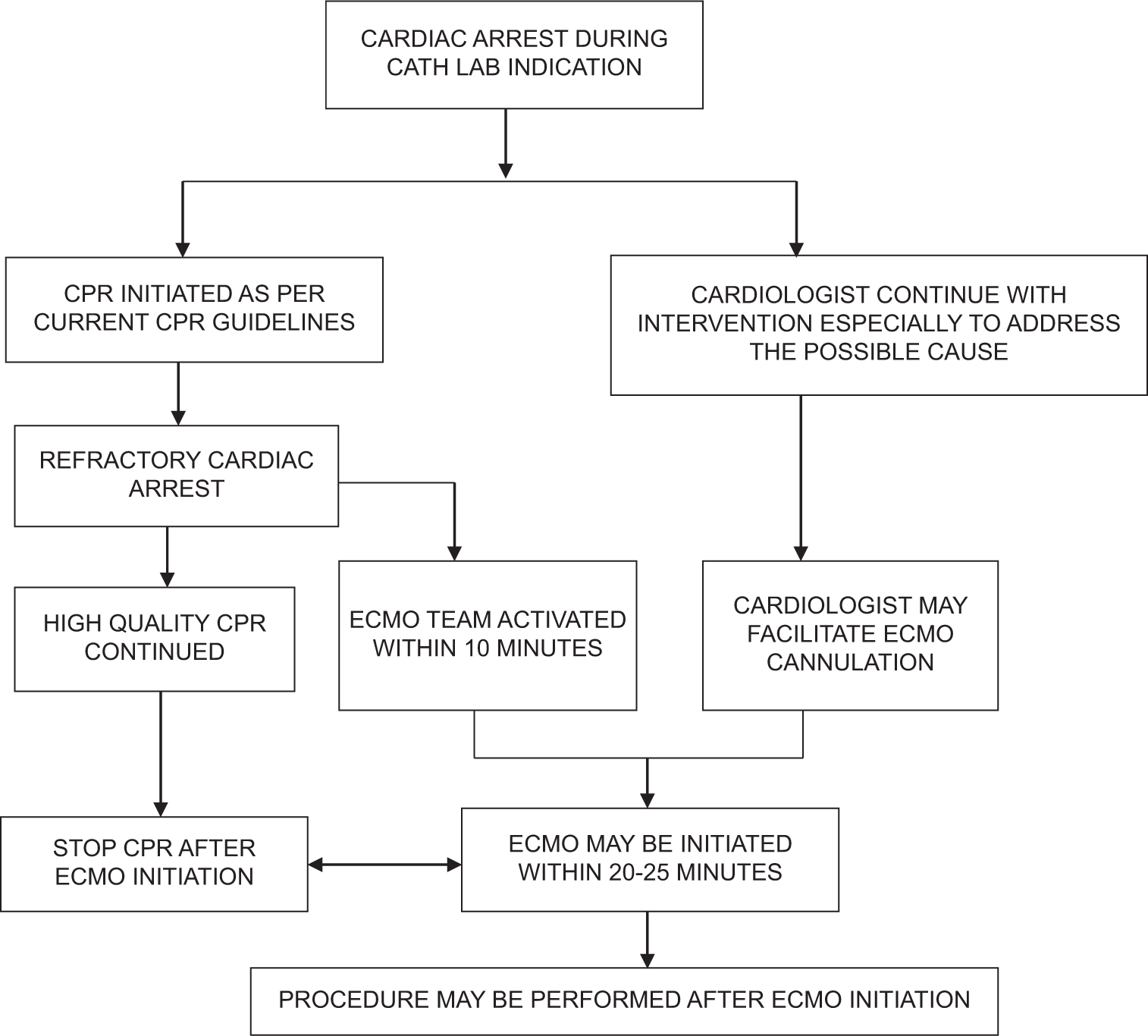
- Proposed ECPR algorithm in catheterization laboratory. (Cardiologist facilitates ECMO cannulation once the team arrives and the circuit is primed). ECPR: extracorporeal cardiopulmonary resuscitation; ECMO: extracorporeal membrane oxygenation; CPR cardiopulmonary resuscitation.
CONCLUSION
The safe and effective use of various configurations of ECMO has increased drastically in the recent past. The increasing technical complexity of catheterization laboratory procedures and selection of high-risk patients may need an additional hemodynamic support in the periprocedural period which sustains the cardiac output and ensures other organ perfusion as well. The use of VA ECMO during catheterization laboratory procedure has several advantages including supporting the other organ perfusion. However, the complications associated with ECMO in general and VA ECMO in particular needs a careful patient selection, vigilant monitoring, and close integration among the team including interventional cardiologist, cardiac anesthesiologist, ECMO specialist. Cardiac surgeon, intensivist, and perfusionist.
Ethical Approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil
References
- Outcomes of Pediatric Patients Undergoing Cardiac Catheterization While on Extracorporeal Membrane Oxygenation. Pediatr Cardiol. 2015;36:625-32.
- [CrossRef] [Google Scholar]
- Utility of Cardiac Catheterization in Pediatric Cardiac Patients on ECMO. Catheter Cardiovasc Interv. 1999;46:62-7.
- [CrossRef] [Google Scholar]
- SCAI Clinical Expert Consensus Statement on the Classification of Cardiogenic Shock: This Document was Endorsed by The American College of Cardiology (ACC), The American Heart Association (AHA), The Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29-37.
- [CrossRef] [Google Scholar]
- Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol. 2015;66:2663-74.
- [CrossRef] [Google Scholar]
- Extracorporeal Membrane Oxygenation during Percutaneous Coronary Intervention in Patients with Coronary Heart Disease. J Extra Corpor Technol. 2020;52:196-202.
- [CrossRef] [Google Scholar]
- Association between Timing of Extracorporeal Membrane Oxygenation and Clinical Outcomes in Refractory Cardiogenic Shock. JACC Cardiovasc Interv. 2021;14:1109-19.
- [CrossRef] [Google Scholar]
- Extra-corporeal Membrane Oxygenation for Refractory Cardiogenic Shock after adult Cardiac Surgery: A Systematic Review and Meta-analysis. J Cardiothorac Surg. 2017;12:55.
- [CrossRef] [Google Scholar]
- New Horizons of Non-emergent Use of Extracorporeal Membranous Oxygenator Support. Ann Transl Med. 2016;4:76.
- [CrossRef] [Google Scholar]
- Outcomes of Extracorporeal Membrane Oxygenation Support for Complex High-Risk Elective Percutaneous Coronary Interventions: A Single-center Experience and Review of the Literature. J Invasive Cardiol. 2018;30:456-60.
- [Google Scholar]
- Prophylactic ECMO during TAVI in Patients with Depressed Left Ventricular Ejection Fraction. Clin Res Cardiol. 2019;108:366-74.
- [CrossRef] [Google Scholar]
- A Case of Successful Mitraclip Implantation in a Patient Having a Large Coaptation Gap under Extracorporeal Membrane Oxygenation (ECMO) Catheter Cardiovasc Interv. 2018;91:827-30.
- [CrossRef] [Google Scholar]
- Venoarterial Extracorporeal Membrane Oxygenation Support for Ventricular Tachycardia Ablation: A Systematic Review. ASAIO J. 2020;66:980-5.
- [CrossRef] [Google Scholar]
- Physiologic Guidance for Percutaneous Coronary Intervention: State of the Evidence. Trends Cardiovasc Med. 2023;33:298-306.
- [CrossRef] [Google Scholar]
- Intravascular Ultrasound-Guided Percutaneous Coronary Intervention: Evidence and Clinical Trials. Interv Cardiol Clin. 2023;12:177-85.
- [CrossRef] [Google Scholar]
- Contemporary Left Main Percutaneous Coronary Intervention: A State-of-the-art Review. Interv Cardiol. 2023;18:e20.
- [CrossRef] [Google Scholar]
- Prognosis of Coronary Heart Disease after Percutaneous Coronary Intervention: A Bibliometric analysis Over the Period 2004-2022. Eur J Med Res. 2023;28:311.
- [CrossRef] [Google Scholar]
- Percutaneous Circulatory Assist Devices for High-risk Coronary Intervention. JACC Cardiovasc Interv. 2015;8:229-44.
- [CrossRef] [Google Scholar]
- Unexpected Deformation of the Right Coronary Artery During Percutaneous Coronary Intervention with Venoarterial Extracorporeal Membrane Oxygenation Combined with Impella: A Case Report. Eur Heart J Case Rep. 2023;7:ytad402.
- [CrossRef] [Google Scholar]
- Prophylactic Veno-arterial Extracorporeal Membrane Oxygenation in Patients Undergoing High-risk Percutaneous Coronary Intervention. Neth Heart J. 2020;28:139-44.
- [CrossRef] [Google Scholar]
- Mechanical Assist Device-Assisted Percutaneous Coronary Intervention: The Use of Impella Versus Extracorporeal Membrane Oxygenation as an Emerging Frontier in Revascularization in Cardiogenic Shock. Cureus. 2023;15:e33372.
- [CrossRef] [Google Scholar]
- Successful Percutaneous Coronary Intervention with Extracorporeal Membrane Oxygenation Support after Right Coronary Artery Dissection in an Eisenmenger Syndrome Patient. Acute Crit Care. 2020;35:46-50.
- [CrossRef] [Google Scholar]
- Protected Complex Percutaneous Coronary Intervention and Transcatheter Aortic Valve Replacement Using Extracorporeal Membrane Oxygenation in a High-risk Frail Patient: A Case Report. J Med Case Rep. 2020;14:163.
- [CrossRef] [Google Scholar]
- Implementation of Extracorporeal Membrane Oxygenation before Primary Percutaneous Coronary Intervention May Improve the Survival of Patients with ST-segment Elevation Myocardial Infarction And Refractory Cardiogenic Shock. Int J Cardiol. 2018;269:45-50.
- [CrossRef] [Google Scholar]
- Short-term Outcomes of Elective High-risk PCI with Extracorporeal Membrane Oxygenation Support: A Single-centre Registry. J Interv Cardiol. 2022;2022:7245384.
- [CrossRef] [Google Scholar]
- Two-year Outcomes after Transcatheter or Surgical Aortic-valve Replacement. N Engl J Med. 2012;366:1686-95.
- [CrossRef] [Google Scholar]
- Transcatheter Aortic-valve Replacement with a Balloon-Expandable Valve in Low-risk Patients. N Engl J Med. 2019;380:1695-705.
- [CrossRef] [Google Scholar]
- Demographics, Procedural Characteristics, and Clinical Outcomes When Cardiogenic Shock Precedes TAVR in the United States. JACC Cardiovasc Interv. 2020;13:1314-25.
- [CrossRef] [Google Scholar]
- Incidence and Predictors of Early and Late Mortality after Transcatheter Aortic Valve Implantation in 663 Patients with Severe Aortic Stenosis. Circulation. 2011;123:299-308.
- [CrossRef] [Google Scholar]
- 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2012;59:1200-54.
- [CrossRef] [Google Scholar]
- Extracorporeal Membrane Oxygenation for Very High-risk Transcatheter Aortic Valve Implantation. Heart Lung Circ. 2014;23:957-62.
- [CrossRef] [Google Scholar]
- Catheter Ablation of Ventricular Tachycardia in Structurally Normal Hearts: Indications, Strategies, and Outcomes-Part I. J Am Coll Cardiol. 2017;70:2909-23.
- [CrossRef] [Google Scholar]
- Catheter Ablation of Ventricular Tachycardia in Structural Heart Disease: Indications, Strategies, and Outcomes-Part II. J Am Coll Cardiol. 2017;70:2924-41.
- [CrossRef] [Google Scholar]
- Acute Hemodynamic Decompensation during Catheter Ablation of Scar-related Ventricular Tachycardia: Incidence, Predictors, and Impact on Mortality. Circ Arrhythm Electrophysiol. 2015;8:68-75.
- [CrossRef] [Google Scholar]
- Outcomes of Catheter Ablation of Ventricular Tachycardia with Mechanical Hemodynamic Support: An Analysis of the Medicare Database. J Cardiovasc Electrophysiol. 2017;28:1295-302.
- [CrossRef] [Google Scholar]
- Extracorporeal Membrane Oxygenation for Hemodynamic Support of Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol. 2016;9:e004492.
- [CrossRef] [Google Scholar]
- Experience in Applied Veno-arterial Extracorporeal Membrane Oxygenation to Support Catheter Ablation of Malignant Ventricular Tachycardia. Int J Cardiol Heart Vasc. 2023;49:101283.
- [CrossRef] [Google Scholar]
- Elective Extracorporeal Membrane Oxygenation Support for High-risk Pediatric Cardiac Catheterization. J Cardiothorac Vasc Anesth. 2019;33:1932-8.
- [CrossRef] [Google Scholar]
- Preoperative Venoarterial Extracorporeal Membrane Oxygenation Slashes Risk Score in Advanced Structural Heart Disease. Ann Thorac Surg. 2018;106:1709-15.
- [CrossRef] [Google Scholar]
- Review of Venoarterial Extracorporeal Membrane Oxygenation and Development of Intracardiac Thrombosis in adult Cardiothoracic Patients. J Extra Corpor Technol. 2016;48:162-7.
- [CrossRef] [Google Scholar]
- Left Ventricular Decompression in Veno-arterial Extracorporeal Membrane Oxygenation. Ann Cardiothorac Surg. 2019;8:9-18.
- [CrossRef] [Google Scholar]
- Factors Associated with Outcomes of Patients on Extracorporeal Membrane Oxygenation Support: A 5-year Cohort Study. Crit Care. 2013;17:R73.
- [CrossRef] [Google Scholar]
- Predictive Factors of Bleeding Events in adults Undergoing Extracorporeal Membrane Oxygenation. Ann Intensive Care. 2016;6:97.
- [CrossRef] [Google Scholar]
- Successful Management of Bleeding Complications in Patients Supported with Extracorporeal Membrane Oxygenation with Primary Respiratory Failure. Perfusion. 2013;28:125-31.
- [CrossRef] [Google Scholar]
- The Impact of Vascular Complications on Survival of Patients on Venoarterial Extracorporeal Membrane Oxygenation. Ann Thorac Surg. 2016;101:1729-34.
- [CrossRef] [Google Scholar]
- Outcomes of Percutaneous Femoral Cannulation for Venoarterial Extracorporeal Membrane Oxygenation Support. Eur Heart J Acute Cardiovasc Care. 2012;1:111-4.
- [CrossRef] [Google Scholar]
- Extracorporeal Membrane Oxygenation Watershed. Circulation. 2014;130:864-5.
- [CrossRef] [Google Scholar]
- Arterial Complications in Patients Undergoing Extracorporeal Membrane Oxygenation Via Femoral Cannulation. Ann Vasc Surg. 2014;28:178-83.
- [CrossRef] [Google Scholar]
- Long-term Outcomes of High-risk Percutaneous Coronary Interventions Under Extracorporeal Membrane Oxygenation Support: An Observational Study. World J Clin Cases. 2022;10:5266-74.
- [CrossRef] [Google Scholar]
- Incidence, Correlates, and Outcome of Cardiac Arrest Associated with Percutaneous Coronary Intervention. Am J Cardiol. 2002;90:1252-4.
- [CrossRef] [Google Scholar]
- Gender Disparities in Patients Undergoing Extracorporeal Cardiopulmonary Resuscitation. Front Cardiovasc Med. 2023;10:1265978.
- [CrossRef] [Google Scholar]
- Sterility and Oxygenator Function in Pre-primed Extracorporeal Membrane Oxygenation: A Prospective Clinical Study. Resusc Plus. 2024;19:100680.
- [CrossRef] [Google Scholar]







