Translate this page into:
Current Status of the Bioresorbable Scaffolds in Coronary Interventions
*Corresponding author: Jyotsna Maddury, Department of Cardiology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India. janaswamyjyotsna@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patnaik A, Maddury J. Current status of the bioresorbable scaffolds in coronary interventions. Indian J Cardiovasc Dis Women 2022;7:214-9.
Drug-eluting stents (DESs) have revolutionized the treatment of coronary atherosclerosis by a dramatic reduction in restenosis. However, limitations such as vascular inflammation, local thrombus formation, loss of vasomotor tone, and restenosis of the stented segment continue to be vexing problems. Occasional late and very late stent thrombosis, stent fatigue fracture, unsuitability of stented segments for future surgical revascularization, impairment of vasomotor tone, and jailing of side branches are unresolved issues with them.[1,2]
To address the above-mentioned problems, bioresorbable scaffolds (BRSs) were designed. Bioabsorbable vascular scaffolds (BVSs) were introduced in 2006, to fix these problems by providing temporary initial support to safeguard the acute luminal gain and disappearance over weeks to allow the restoration of normal vasomotor tone of the vessel. Like DES, neo-intimal proliferation is prevented by the BVS due to the anti-proliferative drug that is used. These novel devices are hailed as revolutionary in concept, as the treated vessel segments have the potential to be good targets for any future surgical revascularization comparable to the native vessels.
THE STRUCTURE AND COMPOSITION OF BVS
The prototype and most widely used scaffold are Absorb BVS which consists of a poly-Llactide frame covered by an a1:1 mixture of Everolimus and poly-D and L-lactide (PLLA) as an amorphous matrix. The device is not radio-opaque, and hence, two radio-opaque markers are embedded at the proximal end. The scaffold has self-expanding property which occurs at body temperature over 20 min but requires storage at 2–8°C. The first version of the device showed a high rate of recoil at 6 months and was replaced by version 1.1. The strut thickness was 150 microns and the design had out-of-phase sinusoidal hoops with links (multi-link design). Its crossing profile was 1.4 mm [Figure 1]. The absorption time is <3 years. In other devices, materials such as poly-salicylic acid, tyrosine polycarbonate magnesium, or other metals are used. PLLA was used in DESolve, Igaki-Tamai, and Amaranth devices. Reva ReZolve used deca-amino-tyrosine polycarbonate material along with Sirolimus. Biotronik company incorporated magnesium in place of PLLA in their devices-absorbable metal stents (AMS) and dreams.[3,4]
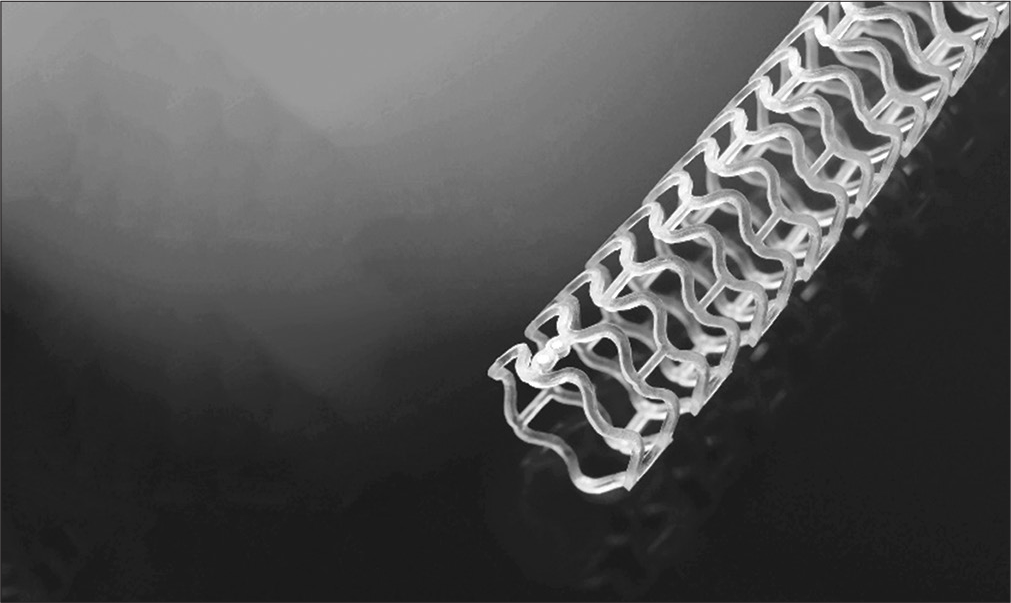
- Bio absorbable vascular scaffolds.
PRECAUTIONS DURING IMPLANTATION OF THE BVS
Prior preparation of the lesion is important. It is advisable to achieve 1:1 lumen to vessel size at the lesion site before implanting the BRS, which can be done by NC balloons in soft lesions or cutting balloon/scoring balloons in fibrotic lesions, or even plaque modification in calcific lesions. It is advisable even to do coronary imaging to confirm the luminal gain with balloon dilatation and to know the cracking of calcific lesions, before using the BVS stent. For overlap stents, the presence of markers on the stent should be taken the help as the scaffold is not visible on the fluoroscopy. The distal marker of the proximal stent should coincide with the proximal marker of the distal stent.
EVALUATION
Non-drug-eluting Igaki-Tamai BVS was the first device of this kind to be tried by Tamai et al. who published their feasibility results in 2000. At 6 months, the restenosis was 10.5%.[5] Among the drug-eluting BVS, Abbott’s BVS was the first to be clinically evaluated and published in 2013.
Absorb trails
ABSORB cohort A was the first-in-man study that used Absorb BVS 1.0, in 30 patients, in vessels sized 3.0–3.5 mm, in lesions shorter than 14 mm. Early recoil at 6 months was the main drawback. Both 2-year and 5-year MACE were 3.4%. These results proved that BVS was comparable to the best available DES of those times.[6] The BVS 1.1 was used in 101 (Cohort B) patients subsequently which yielded better luminal area and good late clinical outcomes – no stent thrombosis at 3 years and MACE rate of 10.1%. These data were supplemented by an EXTENDED study. The stability of MACE rates after resorption (by 2–3 years) even up to 5 years was a notable finding.[7] ABSORB-II compared the BVS with an equivalent metallic stent in 501 patients and ABSORB-III was published in 2015 and enrolled 2008 patients from multiple centers which lead to its approval by FDA for use in the USA. BVS was within the prespecified margin for non-inferiority regarding target lesion failure at 1 year. However, the device thrombosis was double that of the metallic stent group. A meta-analysis that followed also showed a higher incidence of subacute scaffold thrombosis.[8,9] ABSORB IV is almost like an extension of ABSORB-III and its results were announced in TCT in 2018. It showed that the 30-day and 1-year rates of target vessel failure and angina were non-inferior to DES.[10]
Registry data
GHOST-EU registry revealed a definite and probable scaffold thrombosis rate of 2.1% at 6 months and 3.4% at 12 months.[11] BVS registries that included more complex lesions also showed that compared to the second-generation DES, the BVS showed higher 1-year rates for stent thrombosis.[12] However, subsequent observations were more assuring with no higher incidence of ST after BVS implantations, where slight BVS over-sizing and high-pressure post-dilation were followed in all cases.[13,14] An intracoronary imaging follow-up at 3 years in the ABSORB-Japan trial showed very late scaffold thrombosis of 1.6% (vs. none in the cobalt-chromium stent arm) and the mechanism was dismantling of the scaffold.[15] Concurrently, DeSolve, ARTS18AZ, Reva and REZOLVE, Ideal, Xinsorb, and NeoVas – polymer-based BRS were evaluated with promising results. PROGRESS-AMS was the first prospective trial with metallic (magnesium) BVS. Although the acute results were as good as other stents, the radial support was lost very early and resulted in high restenosis rates.[16]
BVS in acute coronary syndromes
The thick struts of BVS have the potential to entrap the thrombotic material during primary PCI in acute coronary syndrome. Despite this apprehension, BVS was tested in the primary PCI and found to have high procedural success and comparable outcomes as with the DES implantations.[17-19]
BVS in complex lesions
Despite initial apprehensions about the use of BVS in true bifurcation lesions, it was found to be safe. Some operators preferred to use inflation pressures lesser than 5 atm to avoid scaffold disruption. Some suggested sequential side branch and main vessel dilatation rather than the kissing balloon technique.[20,21] The BVS if implanted in CTO lesions could have theoretical advantages, but the limitation is the larger crossing profile (1.4 mm). There are a few successful attempts in these complex lesions.[22] In-stent restenosis poses the problem of multiple metal layers when managed with DES. Drug-eluting balloons failed to provide a robust solution for in-stent-restenosis. BVS looks promising to overcome these limitations. However, the actual thickness of the struts of BVS poses a hurdle in negotiating these devices through a stenosed prior stent lumen. However, Moscarella et al. reported, 90 in-stent lesions being tackled using BVS with 100% success. At a 7-month follow-up of their cases, MCE was reported in 12%.[23]
Concerns and unresolved issues
The initial huge enthusiasm for the BVS technology as a replacement for the existing stents in all scenarios met with a sudden halt with reports of subacute and late device thrombosis. After a relook, the need for the meticulous choice of the cases and the modified implantation technique was advocated. Some of the following are other concerns.[3,4]
The struts of BVS are wider and thicker. It is possible that the struts protrude more and affect the laminar flow and may activate platelets. Therefore, better post-dilatation is advocated
Higher incidence of acute device thrombosis is reported; there is a need to validate proper anti-platelet strategy
The anti-proliferative drug itself can influence the reendothelization and healing process
Cost-effectiveness: Some countries have very low-cost effective DES available and BVS have to compete with them
The superiority over current generation DES has to be clearly robust for shifting the choice. In the short-term, both perform equally to each other.
NEW-GENERATION BVS-BRS
Due to high scaffold thrombosis rates, the ABSORB GT1 was withdrawn from the market and newer designs with better materials were brought out [Table 1]. The limitations of the first-generation scaffold were insufficient ductility, low tensile strength, and limited elongation-to-break, poor strength of the polymer, thrombogenicity of lactic acid released during degradation of polymer – all leading to late or very late thrombosis. The new-generation devices are aimed to overcome the above pitfalls. Magmaris, Xinsorb, NeoVas, Firesorb, MeRes 100, Mirage, Bioheart, Fantom Scaffold, Credence BtK (Meril LS), Motiv, etc., are the newly introduced scaffolds that promise to address the prior shortcomings. Moreover, the scaffold strut thinness is 100 µm, relatively thinner than previous BVS with hybrid geometry and PLLA backbone. It has a crossing profile of 1.2 mm with couplets of three radio-opaque markers at each end, which increases visibility. It has good radial strength (1.2 Bars) and low recoil (1.6%) with low balloon overhanging. Other special features for BRS, when compared to previous BVS, are increased radial force, so there are increased chances of over-expansion after deployment and decreased degradation time for the drug. Even the side branch assesses if a good open-cell design is there in the mid portion. These devices are stored at room temperature and under active evaluation.[24] MeRes 100 delivers sirolimus drug (1.25 µg/mm2).
| Scaffold | Strut material | Drug | Company | Accreditation |
|---|---|---|---|---|
| ABSORB-1.0 and 1.1 | PLLA | Everolimus | Abbott Vascular, USA | CE approved, 2011 |
| ESPRIT | FDA approved, 2016 | |||
| DESolve BRS | PLLA | Novolimus/myolimus | Elixir, CA, USA | CE approved, 2016 |
| Magmaris | Biotronik, Germany | CE approved, 2016 | ||
| Igaki-Tamai scaffold | PLLA | Nil | Kyoto, Japan | CE approved |
| AMS | Magnesium Alloy | Paclitaxel | Biotronik, Germany | |
| DREAMS | Magnesium Alloy | Paclitaxel | Biotronik, Germany | |
| ARTS-I and II | PDLLA | Nil | ART, France | CE approved 2015 |
| Ideal-I and II | Polylactide-salicylates | Sirolimus | Xenogenics, USA | |
| REVA-I and II, MOTIV, Fantom | PTD-PC | Sirolimus | Reva Medical, USA | CE approved 2017 |
| Scaffold | ||||
| Medtronic | Medtronic, USA | |||
| MeRes 100 | Meril LS | |||
| CREDENCE BtK | ||||
| Lifetech IBS | Iron | Lifetech, China | ||
| JMDT | JMDT+FUJI, Japan | |||
| Firesorb | Microport, China | |||
| NeoVas | PLLA | Sirolimus | Lepu Medical, China | |
| Xinsorb | PLLA | Sirolimus | HuAnan Biotech, China | |
| Amaranth | PLLA | Nil | Amaranth Medical, USA |
AMS: Absorbable metal stents, PLLA: Poly-D, L-lactide
Optimal implantation technique of BRS
Like BVS, BRS also requires optimal pre-dilatation, optimal vessel and device sizing (balloon to inflate 16 to 20 atm without waist), and adequate post-dilatation (PSP technique – pre-dilatation, sizing, and post-dilatation). To have proper PSP, Meres 100 scaffold provides 2 NC balloons (one balloon is the same size as the scaffold and another 0.25 mm bigger) along with the scaffold. The use of IVUS/OCT can contribute to proper vessel-device sizing and adequacy of the scaffold apposition [Figure 2]. Better avoid vessels <2.5 mm or above 3.75 mm, long lesions, and heavily calcified lesions (learned after BVS usage in calcific lesions). One should try to achieve <10% residual stenosis by optimal post-dilatation.[25-27]
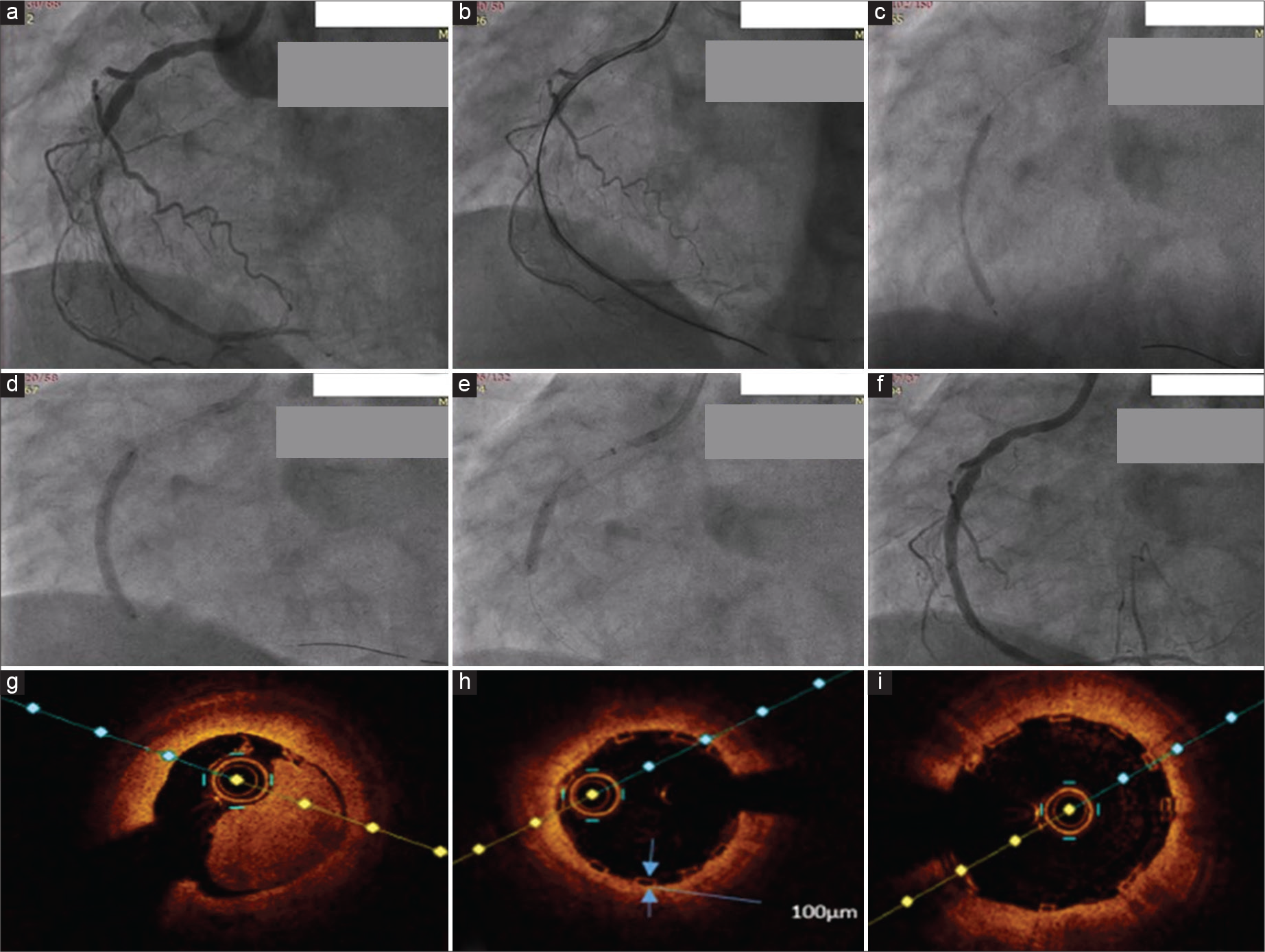
- 3.25 × 40 mm Meres 100 bioresorbable scaffold (BRS) deployment in a near total occlusion of RCA with image guidance. (a) Near total occlusion of RCA. (b) Lesion crossed with guidewire with guidezilla support. (c) Lesion dilatation done with 2.5 and 3.25 mm balloons. (d) 3.25*40 mm BRS deployed. (e) Post-dilatation with 3.5 mm balloon. (f) Final angiographic picture. (g) Initial OCT run. (h) OCT run after deploying BRS, showing strut thickness of 100 µm. (i) OCT run after post-dilatation showing adequate stent expansion and apposition.
EVALUATION
In the initial phase of BRS usage, preferred to use in a young patient with two vessel lesion or two BRS in a single vessel without overlapping and in selected primary PCI cases.
Studies
MERES 1 TRAIL – Seth et al. reported the 3 years clinical outcome of Meres 100, in 108 patients with cumulative MACE of 1.87%.[28] This MACE was due to TLR, no cardiac deaths or MI, or scaffold thrombosis.
HYBRID STENTING
Even though the resolution of a stent with the restoration of vasomotor function is an attractive feature of BRS, it is not possible that in every coronary lesion BRS. Hence, the concept of hybrid stenting has come (BRS in proximal lesions, either DES or DEB in distal diffuse lesions). The feature favoring this concept is still metal burden is less than full jacking with metallic DES in long lesions.[29]
Use in other arenas
The encouraging results in coronary disease lead to their use in peripheral artery disease and in the biliary and gastrointestinal system.
CONCLUSION
BVS is a novel concept with promising clinical data, especially with the new generation of devices. When there is a need for a good late vessel lumen with restored vasomotion BVS is the answer. The initial setbacks are fairly addressed in the new-generation devices and there are improvements in implantation techniques. With maturity in technology and clinical experience, BVS is expected to re-bounce as a promising strategy in interventional cardiology.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897-907.
- [CrossRef] [PubMed] [Google Scholar]
- Stent thrombosis with drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393-402.
- [CrossRef] [PubMed] [Google Scholar]
- Bioresorbable vascular scaffolds: Design, clinical trials and current applications. Coron Artery Dis. 2016;27:151-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bioresorbable vascular scaffolds in the clinical setting. Interv Cardiol. 2013;6:639-46.
- [CrossRef] [Google Scholar]
- Initial and 6 months results of biodegradable poly-1-lactic acid coronary stents in humans. Circulation. 2000;102:399-404.
- [CrossRef] [PubMed] [Google Scholar]
- Five-year clinical and functional Multislice computed tomography results after coronary implantation of the fully resorb able polymeric Everolimus-eluting scaffold in patients with de novo coronary artery disease: The ABSORB cohort a trial. JACC Cardiovasc Interv. 2013;6:999-1009.
- [CrossRef] [PubMed] [Google Scholar]
- ABSORB EXTEND investigators. The ABSORB EXTEND study: Preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015;10:1396-401.
- [CrossRef] [PubMed] [Google Scholar]
- Bioresorbable vascular scaffolds-will promise becomes reality? N Eng J Med. 2015;373:n1969-71.
- [CrossRef] [PubMed] [Google Scholar]
- ABSORB III investigators. Everolimuseluting bioresorbable scaffolds for coronary artery disease. N Eng J Med. 2015;373:1905-15.
- [CrossRef] [PubMed] [Google Scholar]
- Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1 year results from ABSORB IV randomized trial. Lancet. 2018;392:1530-40.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous coronary intervention with Everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicenter GHOST-EU registry. EuroIntervention. 2015;10:1144-53.
- [CrossRef] [PubMed] [Google Scholar]
- Initial experience and clinical evaluation of the absorb bioresorbable vascular scaffold (BVS) in real-world practice: The AMC single center real world PCI registry. EuroIntervention. 2015;10:1160-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of early clinical outcomes between ABSORB bioresorbable vascular scaffold and Everolimuseluting stent implantation in a real-world population. Catheter Cardiovasc Interv. 2015;8:E10-5.
- [CrossRef] [PubMed] [Google Scholar]
- ABSORB biodegradable stents versus second-generation metal stents: A comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc Interv. 2014;7:741-59.
- [CrossRef] [PubMed] [Google Scholar]
- Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an Everolimus-eluting bioresorbable scaffold and an Everolimuseluting metallic stent: Insights from the ABSORB Japan trial. EuroIntervention. 2016;12:1090-101.
- [CrossRef] [PubMed] [Google Scholar]
- PROGRESS-AMS (Clinical Performance angiographic results of coronary stenting with absorbable metal stents) investigators: Early and long-term intravascular ultrasound and angiographic findings after magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv. 2009;2:312-20.
- [CrossRef] [PubMed] [Google Scholar]
- Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction: A prospective multicenter study “Prague 19”. Eur Heart J. 2014;35:787-94.
- [CrossRef] [PubMed] [Google Scholar]
- Absorb bioresorbable vascular scaffold versus Everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: The BVS-EXAMINATION study. JACC Cardiovasc Interv. 2015;8:189-97.
- [CrossRef] [PubMed] [Google Scholar]
- Everolimus-eluting bioresorbable stent vs. durable polymer Everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37:229-40.
- [CrossRef] [PubMed] [Google Scholar]
- Lessons from the GHOST-EU registry. EuroIntervention. 2015;11:V170-4.
- [CrossRef] [PubMed] [Google Scholar]
- Absorb Everolimus-eluting bioresorbable scaffolds in coronary bifurcations: A bench study of deployment, side branch dilatation and post-dilatation strategies. EuroIntervention. 2015;10:1169-77.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes and computed tomography scan follow-up of bioresorbable vascular scaffold for the percutaneous treatment of chronic total coronary artery occlusion. Am J Cardiol. 2015;115:1487-93.
- [CrossRef] [PubMed] [Google Scholar]
- Bioresorbable vascular scaffold implantation for the treatment of coronary in-stent restenosis: Results from a multicenter Italian experience. Int J Cardiol. 2015;199:366-72.
- [CrossRef] [PubMed] [Google Scholar]
- Bioresorbable scaffolds: History and current knowledge. Cardiol Plus. 2016;1:20-5.
- [CrossRef] [Google Scholar]
- Bioresorbable coronary scaffold thrombosis: Multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol. 2016;67:921-31.
- [CrossRef] [PubMed] [Google Scholar]
- Report of an ESC-EAPCI task force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: Executive summary. Eur Heart J. 2018;39:1591-601.
- [CrossRef] [PubMed] [Google Scholar]
- Three-year clinical and two-year multimodality imaging outcomes of a thin-strut sirolimuseluting bioresorbable vascular scaffold: MeRes-1 trial. Eurointevention. 2019;15:607-14.
- [CrossRef] [PubMed] [Google Scholar]
- Hybrid percutaneous coronary intervention with bioresorbable vascular scaffolds in combination with drug-eluting stents or drug-coated balloons for complex coronary lesions. J Am Coll Cardiol Interv. 2017;10:539-47.
- [CrossRef] [PubMed] [Google Scholar]







