Translate this page into:
Assessment of Inflammatory Markers among Hypertensive Women in a Nigerian Population
*Corresponding author: Emmanuel Akokhamen Omon, Department of Medical Laboratory Science, College of Medicine and Health Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria. omonea@abuad.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Omon EA, Oluboyo AO, Odewusi OO. Assessment of Inflammatory Markers among Hypertensive Women in a Nigerian Population. Indian J Cardiovasc Dis Women. doi: 10.25259/ IJCDW_9_2024
Abstract
Objectives
Essential hypertension (HTN), a leading cause of preventable death and disability worldwide, remains a major global risk factor for cardiovascular diseases. The study assessed the inflammatory markers of hypertensive women in a Nigerian population.
Materials and Methods
Two hundred individuals were recruited for this study comprising 80 hypertensive women on treatment, 50 newly diagnosed hypertensive women, and 70 non-hypertensive women (control). Human interferon-gamma (IFN-γ) and transforming growth factor-beta 1 (TGF-β1) as inflammatory markers were analyzed using Enzyme-Linked Immunosorbent Assay.
Results
The results showed that IFN-γ and TGF-β1 were significantly higher in hypertensive subjects compared with control (P < 0.05). IFN-γ and TGF-β1 were significantly higher (P < 0.05) in hypertensive drug naïve subjects compared with subjects on antihypertensive drugs. Combined use of amlodipine/lisinopril, lisinopril/ metoprolol resulted in significant decrease (P < 0.05) in IFN-γ and TGF-β1 compared with hypertensive patients who used amlodipine and lisinopril alone. There was significant increase (P < 0.05) in IFN-γ and TGF-β1 in age group 51–65 years compared with age group 20–35 years, but there was no significant difference (P > 0.05) in both parameters studied with respect to duration of illness.
Conclusion
The study concluded that hypertensive subjects undergo inflammation marked by increase in inflammatory markers despite the possible mitigating effect of their antihypertensive medication. Therefore, management of HTN in addition to use of antihypertensive drugs should focus on lifestyle modification, Dietary Approaches to Stop HTN, and increased physical activity.
Keywords
Antioxidants
Essential hypertension
High blood pressure
Inflammation
Oxidative stress
ABSTRACT IMAGE
INTRODUCTION
High blood pressure (BP), the hallmark of hypertension (HTN), causes vascular damage, including altered contractility, endothelial dysfunction, and vascular remodeling. According to most recent classification, a constant systolic BP (SBP) larger than or equal to 130 mmHg and diastolic BP (DBP) more than or equal to 80 mmHg are benchmarks used in the diagnosis of HTN.[1] BP levels vary widely in the population and generally rise with advancing age. There are two classifications of HTN: Secondary HTN and primary or essential HTN. Ninety to ninety-five percentages of cases of HTN are primary or essential HTN, which is generally described as high BP caused by genetic factors and nonspecific lifestyle factors including obesity, smoking, alcoholism, physical inactivity, occupation, etc.[2] Secondary HTN responsible for 5–10% of HTN cases is characterized as high BP with a known cause, such as endocrine disorders, the use of birth control pills, narrowing of kidney arteries, or chronic kidney disease.[3]
Inflammation is a multifaceted process that involves multiple cells and signaling proteins that shield the body from infections and foreign objects. An infection triggers a series of events that draw inflammatory cells, which then release chemokines and cytokines.[4] Biomarkers known as inflammatory markers are employed in clinical settings to evaluate patients for the existence or lack of an ongoing inflammatory disease process, as well as the activity of known disorders. Inflammatory markers are crucial for numerous cellular functions, including immune response, migration, apoptosis, differentiation, growth, and tissue homeostasis maintenance.[5] Human interferon-gamma (IFN-γ) is a pleiotropic cytokine that stimulates the removal of infections and activates the immune response in an inflammatory environment and prevents tissue injury and immune system overactivity.[6] Transforming growth factor-beta (TGF-β) is a dimeric protein that is linked to disulfides and weighs 25 kDa available in three different isoforms: TGF-β1, TGF-β2, and TGF-β3. TGF-β1 functions as an anti-inflammatory regulator and influences numerous clinical processes, including regulatory mechanisms related to embryonic development, immune system suppression, cellular proliferation and differentiation, angiogenesis, fibrosis, and the regulation of tissue regeneration and repair following damage.[7]
Globally, HTN is a major risk factor for cardiovascular diseases and a leading cause of avoidable mortality and disability. The pathogenesis of HTN involves inflammation as a central component. It influences the onset, course, and progression of HTN in addition to causing end-organ damage. Furthermore, inflammation generates reactive oxygen species (ROS) that lead to endothelial dysfunction and atherosclerosis.[8] This study therefore aimed at assessing the inflammatory markers of hypertensive women in a Nigerian population.
Aims and objectives
The aim of this study was to assess the inflammatory markers of hypertensive women in a Nigerian population. The specific objectives include to assess;
The inflammatory of hypertensive women compared with non-hypertensive women (control)
The inflammatory markers of hypertensive women not on treatment compared to those on treatment and type of antihypertensive drug used
The inflammatory markers of hypertensive women in relation to age
The correlation between inflammatory markers in hypertensive women.
MATERIALS AND METHODS
Study area
This research was done in Ikere, Ekiti State. Ikere is the principal city and second most populous city in Ekiti State, Southern Nigeria. The region is located between longitudes 50 14’ East of the Greenwich meridian and latitudes 70 30’ North of the equator with a land area of 262 square km with a population density of 778.3/km2.
Study design
This research employed a case–control study design. Females with HTN (with or without treatment) between the ages of 20 and 65 years were recruited for this study.
Ethical approval
Ethical approval for this research was obtained from the Health Research Ethical Committee, Afe Babalola University Ado-Ekiti (ABUAD), Ekiti State (ABUADHREC/26/07/2023/195). Informed consent was sought and obtained from all participants before sample collection.
Sample size
Sample size was calculated using the prevalence formula; n = z2pq/d2
Where; n = the required sample size
z = is a constant given as 1.96 which corresponds to the 95% confidence level P = Prevalence (9.3%)[9]
q = 1.0 – p
d = acceptable error (5%)
n = (1.96)2 × 0.056 × (1 – 0.056)/(0.05)2
n = ≈130
A total of 200 individuals were recruited for this study comprising 130 hypertensive women (subjects) and 70 non-hypertensive women (control). Furthermore, the subject group was divided into two groups comprising 80 hypertensive women on antihypertensive drugs and 50 newly diagnosed hypertensive women.
Inclusion and exclusion criteria
Hypertensive women with or without treatment (antihypertensive agents) who gave their consent were included in this study. Pregnant women and nursing mothers, women with underlining health conditions, elderly women, those taking vitamins or mineral supplement, and those who did not give their consent were excluded from the study.
Anthropometric measurements
BP was measured using mercury sphygmomanometer.
Body mass index (BMI): Weight measurement was done using a weighing scale, height was measured using a meter rule, while BMI was calculated using the formula: Weight (kg)/height (m2).
Waist circumference (WC), wrist circumference, and hip circumference (HC) were measured using measuring tape.
Blood sample collection
For each subjects, 5 mLs of blood sample was collected through venipuncture and dispensed into a plain bottle without any anticoagulant. It was allowed to stand for 1 h to clot and separated by centrifugation at 12,000 rpm for 5 min. After centrifugation, the serum was carefully withdrawn into a pre-labeled tube and stored at –20°C.
Sample analysis
Human IFN-γ and TGF-β1 were analyzed using Enzyme-Linked Immunosorbent Assay kit (Elabscience Biotechnology Inc., USA) according to the manufacturer’s instructions.
Statistical analysis
The Statistical Package for the Social Sciences version 25.0 was used to analyze the data generated from this study. The mean ± standard deviation was used to express all the data. The one-way analysis of variance was employed to assess group differences. Pearson’s correlation coefficient was used to estimate correlations between parameters. The threshold for acceptable significance was set at P < 0.05. All results were presented in tables, and figures.
RESULTS
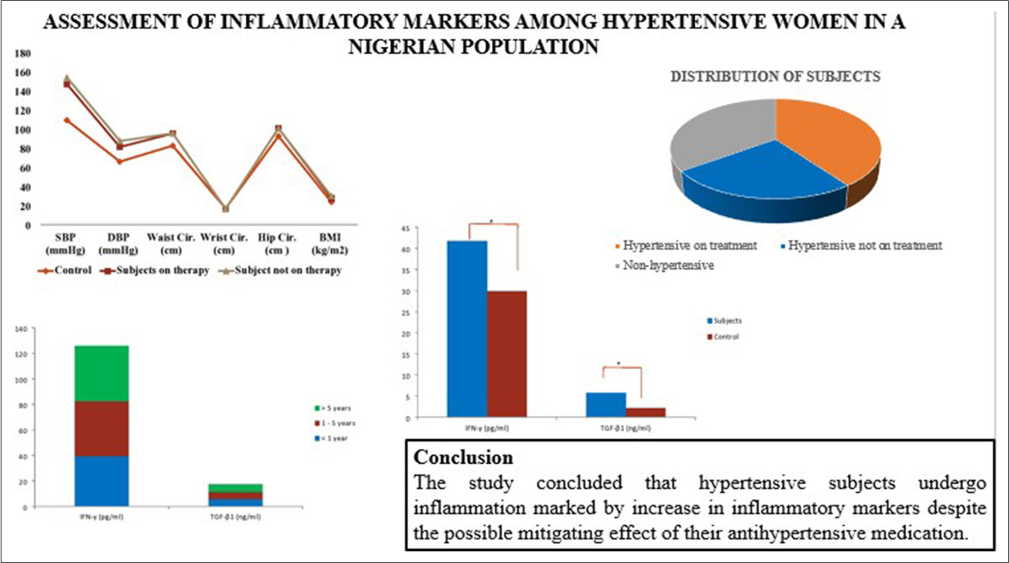
Figure 1 showed the anthropometric parameters of the subjects and healthy control group. The results obtained showed that SBP (mmHg), DBP (mmHg), WC, hip ratio, and BMI (kg/m2) were significantly higher (P < 0.05) in hypertensive subjects on antihypertensive drugs and drug naïve hypertensive subjects compared with healthy control. There was no significant difference (P > 0.05) in SBP (mmHg), DBP (mmHg), WC, hip ratio, and BMI (kg/m2) of hypertensive subjects on antihypertensive drugs compared with drug naïve hypertensive subjects. There was no significant difference (P > 0.05) in the wrist circumference of hypertensive subjects (with or without antihypertensive drugs) compared with healthy control.
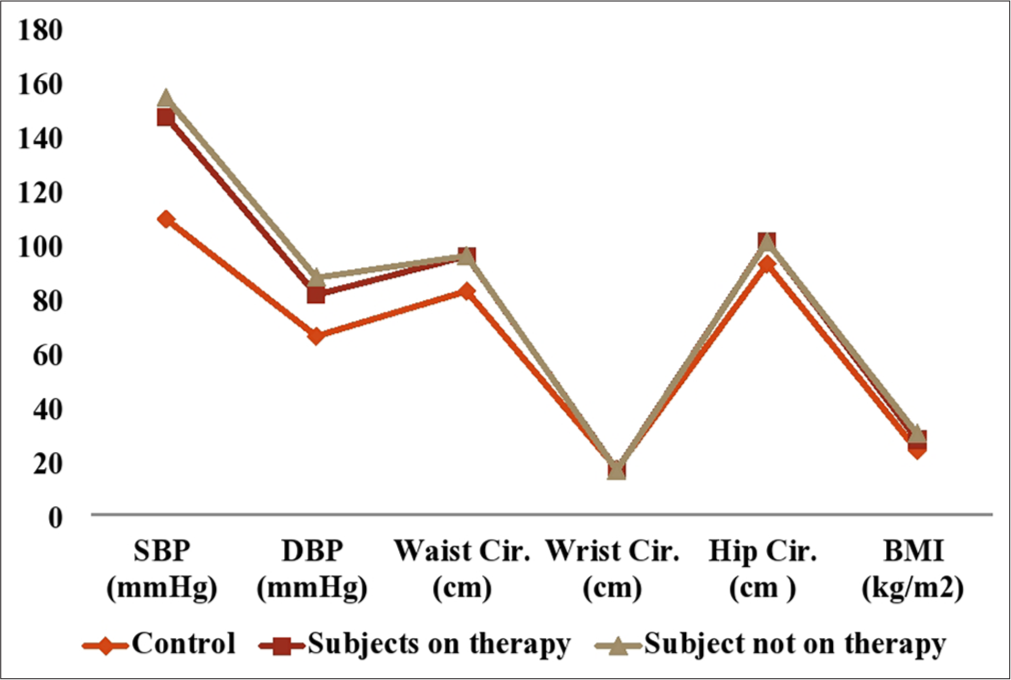
- Anthropometric parameters of hypertensive subjects and control. SBP: Systolic blood pressure, DBP: Diastolic blood pressure, BMI: Body mass index.
Figure 2 showed that IFN-γ and TGF-β1 were significantly higher (P < 0.05) in hypertensive subjects compared with control. Figure 3 showed that inflammation marked by increase in human IFN-γ and TGF-β1 were more pronounce in subjects with HTN >5 years compared with hypertensive subjects <1 year. However, the differences were not statistically significant (P > 0.05) in all the parameters studied.
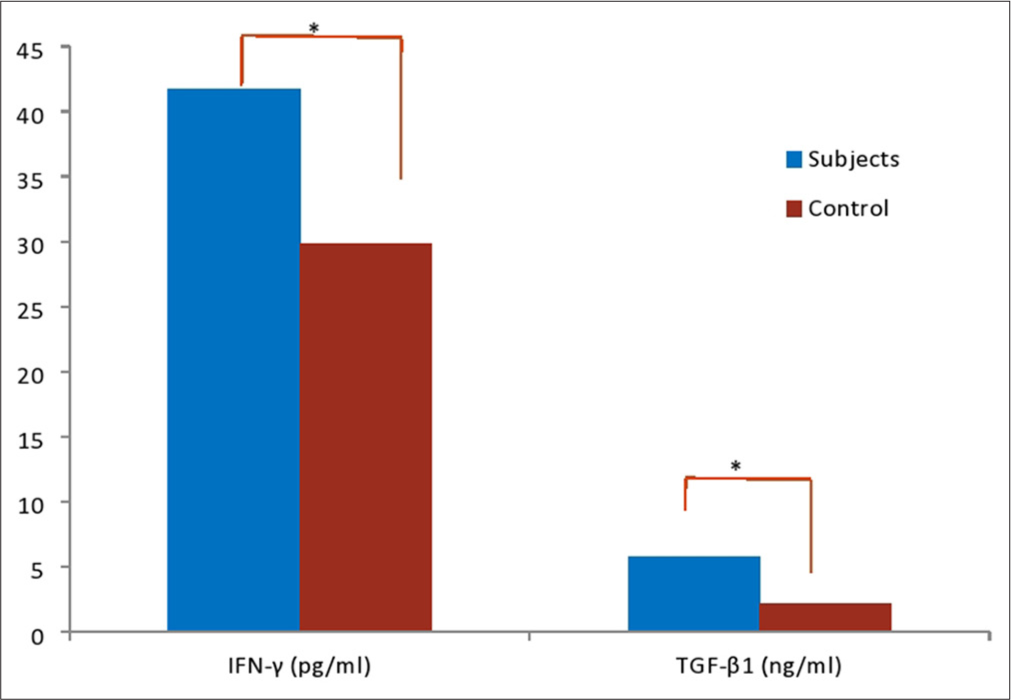
- Inflammatory, oxidative stress, and antioxidants markers of subjects and control. *: Values are significant at P<0.05. IFN: Human interferon gamma, TGF: Tumour growth necrosis factor.
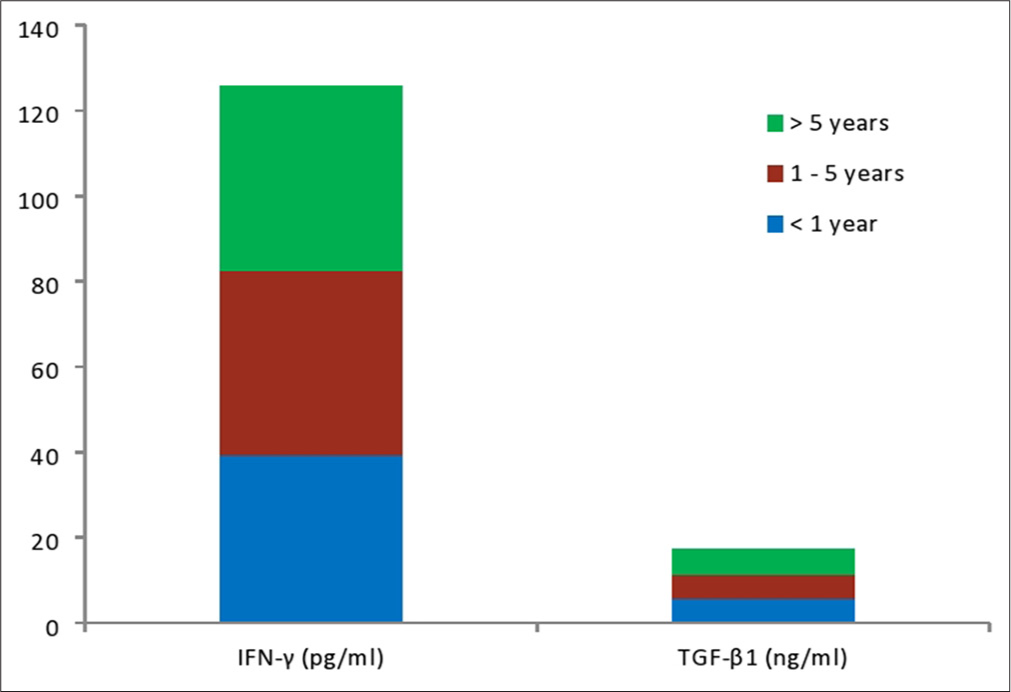
- Inflammatory, oxidative stress, and antioxidants markers of subjects according to duration of illness.
Table 1 showed that IFN-γ and TGF-β1 were significantly higher in antihypertensive drug naïve subjects compared with subjects on antihypertensive drugs (P < 0.05). Furthermore, IFN-γ and TGF-β1 were significantly higher (P < 0.05) in drug naïve subjects and subjects on antihypertensive drugs compared with the control group, respectively. Table 2 showed that combined use of amlodipine/lisinopril, lisinopril/ metoprolol resulted in statistical significant decrease (P < 0.05) in IFN-γ and TGF-β1 compared with hypertensive patients who used amlodipine and lisinopril alone. On the other hand, the combine use of amlodipine/diuretics showed subjects (with or without treatment) compared with healthy non-significant decrease (P > 0.05) in IFN-γ and TGF-β1 compared with hypertensive patients who used amlodipine or lisinopril alone. There was no significant difference (P > 0.05) in both parameters studied in hypertensive subjects who used amlodipine alone compared with lisinopril alone, and combine use of amlodipine/lisinopril compared with lisinopril/metoprolol, respectively. Table 3 showed that IFN-γ and TGF-β1 were significantly higher (P < 0.05) in age group 51–65 years compared to age group 20–35 years and 36–50 years, respectively. Table 4 showed that there was significant positive correlation between IFN-γ and TGF-β1 (r = 0.872, P = 0.000) in hypertensive subjects.
| Variables | Subjects on drugs Mean±SD | Drug naïve subjects Mean±SD | Control Mean±SD |
F-value | P-value |
|---|---|---|---|---|---|
| IFN-γ(pg/mL) | 48.45±13.83a | 79.01±23.15b | 29.82±8.67c | 6.308 | 0.000 |
| TGF-β1 (ng/mL) | 6.55±2.37a | 9.79±1.92b | 2.11±1.11c | 8.897 | 0.000 |
| Variables | Amlodipine Mean±SD | Lisinopril Mean±SD | Amlodipine/Diuretics Mean±SD | Amlodipine/Lisinopril Mean±SD | Lisinopril/Metoprolol Mean±SD | P-value |
|---|---|---|---|---|---|---|
| IFN-γ(pg/mL) | 47.51±12.91a | 39.41±10.22a | 37.36±6.33a | 36.54±8.22b | 35.51±7.24b | 0.010 |
| TGF-β1 (ng/mL) | 7.24±3.62a | 6.29±3.38a | 5.95±1.74a | 5.16±1.03b | 5.13±1.28b | 0.005 |
| Variables | 20–35 years Mean±SD |
36–50 years Mean±SD |
51–65 years Mean±SD |
F-value | P-value |
|---|---|---|---|---|---|
| IFN-γ(pg/mL) | 36.46±14.08a | 35.79±7.24a | 47.18±12.16b | 2.320 | 0.037 |
| TGF-β1 (ng/mL) | 4.72±1.48a | 4.86±1.56a | 6.22±2.60b | 5.556 | 0.000 |
| IFN-γ | TGF-β1 | |
|---|---|---|
| IFN-γ | ||
| Pearson correlation | 0.872** | |
| Significance (two-tailed) | 0.000 | |
| TGF-β1 | ||
| Pearson correlation | 0.872** | |
| Significance (two-tailed) | 0.000 |
DISCUSSION
In this study, we observed significant increase (P < 0.05) in SBP and DBP of hypertensive subjects on treatment and treatment of naïve hypertensive subjects compared with healthy control. Characterized by a persistent rise in SBP and DBP, respectively, essential HTN is a significant risk factor for morbidity and mortality from coronary heart disease, stroke, and kidney disease. Increased SBP can lead to kidney injury by enhancing the oxidative stress response and influencing the myogenic contractile effects in hypertensive individuals.[10] This result is consistent with previous studies[10-12] which confirmed the significant increase in SBP and DBP of hypertensive subjects. The study findings indicate that antihypertensive medications can halt the progressive course of HTN, as seen by the non-significant difference (P > 0.05) in SBP and DBP between hypertensive subjects on treatment and those who were not on treatment which is in tandem with previous studies.[11,12]
In this study, BMI was significantly higher in hypertensive subjects (with or without treatment) compared with healthy control, but no significant difference was observed in BMI of hypertensive subjects on treatment compared with treatment naïve hypertensive subjects. This finding implies that obesity and being overweight may both cause and contributes to the development of essential HTN. The hypothesized mechanisms linking HTN with obesity are multifaceted, some of which include the overactivity of the sympathetic nervous system, sodium retention that increases renal reabsorption, changes in cytokines derived from adipose tissue, activation of the renin-angiotensinaldosterone system, insulin resistance, and structural and functional changes in the kidneys.[13] To achieve the desired effect, treatment for obesity in HTN should focus on lifestyle modification through increased regular physical activity, dietary changes, caloric restriction, and behavioral changes.[14] This finding agrees with earlier studies which reported significantly higher BMI in hypertensive subjects compared with healthy control.[13-15]
There was significant increase in WC and HC of hypertensive control. WC and HC have been associated with high BP in females.[16] The buildup of visceral adipose tissue in the abdomen is one of the processes connecting WC to elevated BP.[17] Our findings imply that the accumulation of abdominal fat may increase SBP and DBP in the presence or absence of BMI rise since WC is a simple marker for visceral and subcutaneous abdominal fat. Weight control is crucial in the prevention and management of essential HTN.[18] Therefore, BP management that targets BMI gain alone may overlook people at risk of higher BP having elevated WC without significant increase in BMI. This finding agrees with the previous studies in which significant increase in WC of hypertensive subjects (with or without treatment) compared with healthy control have been reported.[17-19] Our findings proposed that, when assessing the cardiometabolic risk associated with fat distribution, WC and HC should be measured regardless of BMI. Given the rising prevalence of HTN and the associated cerebrovascular and cardiovascular diseases globally, the management of obesity, particularly abdominal obesity, needs to be reinforced in order to prevent and manage existing HTN.[16]
There was a significant increase in TGF-β of hypertensive subjects (with or without treatment) compared with control (P < 0.05). Numerous factors, such as increased fluid shear stress, genetic TGF-β deoxyribonucleic acid polymorphism, increased renin release from juxtaglomerular cells in the kidney, stimulating the expression of endothelin mRNA in the vascular endothelium, regulation expression of angiotensin II, and increased systemic BP may contribute to increased TGF-β levels in hypertensive subjects.[20] In human adipose tissue, there has been evidence of increased TGF-β expression as well as a connection between TGF-β and BMI.[21] It has been observed that TGF-β is mostly promoted by obesity during in vivo expression in hypertensive patients, and that overproduction of TGF-β may have a role in the long-term effects of HTN.[21] As demonstrated by the increase in WC and BMI of hypertensive subjects in this study, our findings thus seem consistent with an adipose tissue origin for TGF-β and tend to implicate the adipose tissue as a common precursor of TGF-β circulating levels. TGF-β1 has also been shown to stimulate the growth of vascular smooth muscle, thickening the layer of vascular muscle and improving contraction; these effects raise peripheral circulation resistance and encourage the development of HTN.[22] This finding agrees with previous studies[20-22] which reported significant increase in TGF-β of hypertensive subjects (with or without treatment) compared with healthy normotensive subjects.
In this study, we recorded a significant increase in IFN-γ of hypertensive subjects (with or without treatment) compared with healthy control. IFN-γ is essential for the development and progression of HTN. The development of essential HTN and the likelihood of having high SBP have been associated with T-cell activation, which is marked by increased IFN-γ production.[23] IFN-γ may be used as a diagnostic marker due to its association with essential HTN.[24] IFN-γ generated by activated T-cells causes endothelial dysfunction and oxidative stress injury, which both contribute to HTN.[25] T-cells that secrete interferon may interact with RAAS by controlling the synthesis of angiotensinogen, which raises BP.[24] In addition, immune cells including neutrophils, macrophages, dendritic cells, T-cells, and NK cells have all been linked to the process of HTN, suggesting a possible relationship between increased IFN-γ production and HTN.[25] This finding agrees with previous studies which reported significantly increased IFN-γ in hypertensive subjects.[23-25]
In this study, treatment was found to significantly decrease TGF-β and IFN-γ in hypertensive subjects on treatment compared with hypertensive subjects not on treatment (P < 0.05). It has been demonstrated that treating HTN with β-blockers (like metoprolol), calcium channel blockers (like amlodipine), or angiotensin receptor blockers (e.g., losartan) lowers BP and oxidative damage markers.[26] Antihypertensive medications improve endothelial function, reverse vascular remodeling, and lower risk of cardiovascular complications through their antioxidant, anti-inflammatory, anti-atherosclerotic, or anti-fibrinolytic properties. These actions could reverse the anatomical alterations in the small and large arteries, lower peripheral vascular resistance, and enhance arterial function.[27] Our observation that hypertensive subjects on antihypertensive treatment had reduced parameters of oxidative stress and inflammation, and enhanced activity of antioxidant enzymes agrees with previous studies which reported changes in inflammatory, oxidative stress, and antioxidant markers of hypertensive subjects on treatment.[26-28]
Furthermore, the combine use of amlodipine/lisinopril and lisinopril/losartan resulted in statistical significant decrease in IFN-γ and TGF-β levels compared with hypertensive patients who used amlodipine or lisinopril alone. These results suggest that combining antihypertensive drugs from distinct and complementary classes results in a synergistic effect of lowering BP, which consequently leads to enhanced antihypertensive efficacy.[29] The renin-angiotensin system is implicated in the development of HTN and damage to several organs. Thus, angiotensin-converting enzyme-inhibitors such as lisinopril and angiotensin II receptor blockers like losartan are essential for treating HTN and related cardiovascular diseases. These medications not only significantly reduce BP but also the morbidity and mortality linked to cardiovascular disease among hypertensive patients.[30] This result is consistent with other research which reported that the use of combination medications for the treatment of HTN results in a greater antihypertensive effect.[28-30] However, caution must be taken to avoid drug combination that could cause adverse or toxic effect to the patients.
In this study, we observed significant increase in IFN-γ and TGF-β1with increase in age of hypertensive subjects with or without treatment. The complicated and heterogeneous nature of aging, which is marked by a decline in sex hormone levels and increase in visceral adipose tissue, may account for the increase in inflammatory and oxidative stress markers with age.[31] Furthermore, IFN-γ correlated positively with TGFβ1 indicating inflammation and oxidative stress in HTN. This finding agrees with Kaur et al.,[32] who reported that as a result of altered interactions between the antioxidant system and inflammatory events, disturbed homeostasis raises oxidative stress levels, which ultimately cause HTN and its related complications. Correlation analysis confirmed the role of inflammation in compromised endothelial function and potential effect to critical organs linked to HTN.
Limitation of the study
The sample size may be limited, affecting generalizability to the broader Nigerian population. The cross-sectional case-controlled design does not allow for causal inferences or longitudinal tracking of changes in inflammatory markers. Furthermore, potential confounding variables like diet, physical activity, or socioeconomic status may not have been fully accounted for.
CONCLUSION
The study concludes that hypertensive subjects undergo inflammation marked by increase in IFN-γ and TGF-β1despite the possible mitigating effect of their antihypertensive medication. Therefore, management of HTN in addition to use of antihypertensive drugs should focus on lifestyle modification, Dietary Approaches to Stop HTN, and increased physical activity.
Ethical approval
The research/study was approved by the Institutional Review Board at Health Research Ethical Committee, Afe Babalola University Ado-Ekiti (ABUAD), Ekiti State, number ABUADHREC/26/07/2023/195, dated July 26, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Stages of Hypertension. 2021. Available from: https://www.health.harvard.edu/heart/health/reading/the/new/blood/pressure/guidelines [Last accessed on 2024 Mar 04]
- [Google Scholar]
- The Global Epidemiology of Hypertension. Nat Rev Nephrol. 2020;16:223-37.
- [CrossRef] [PubMed] [Google Scholar]
- Physical Activity, Obesity, and Hypertension among Adults in a Rapidly Urbanised City. Int J Hypertens. 2021;2021:9982562.
- [CrossRef] [PubMed] [Google Scholar]
- IL-6 in Inflammation, Autoimmunity and Cancer. Int Immunol. 2021;33:127-48.
- [CrossRef] [PubMed] [Google Scholar]
- Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice-Which to Use Regarding Disease Outcomes? Antioxidants (Basel). 2021;10:414.
- [CrossRef] [PubMed] [Google Scholar]
- Structure of the IFNγ Receptor Complex Guides Design of Biased Agonists. Nature. 2019;567:56-60.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of the TGF-β Superfamily in Myocardial Infarction. Front Cardiovasc Med. 2019;6:140-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Controlled Blood Pressure among Hypertensive Patients and Determinants of Hypertensive Complications in a Nigerian Population. Int J Med Health Dev. 2022;27:226-32.
- [CrossRef] [Google Scholar]
- Hypertension and its Correlates among in-School Adolescents in Ekiti State, South-West, Nigeria. Asian J Med Sci. 2021;8:144-9.
- [Google Scholar]
- Superoxide Dismutase as a Protective Factor for Microalbuminuria in Hypertensive Patients. Sci Rep. 2022;12:20432.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of Renal Biomarkers, Electrolyte Imbalances and Vitamin D Levels in Hypertensive Subjects. Med Int (Lond). 2025;5:20.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of Oxidative Stress and Protein Modification in Essential Hypertensive Subjects. Sokoto J Med Lab Sci. 2023;8:36-45.
- [CrossRef] [Google Scholar]
- The Effect of Body Mass Index and its Interaction with Family History on Hypertension: A Case-Control Study. Clin Hypertens. 2019;25:6.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship Between Clinical Features and Body Mass Index among Hypertensive Patients: A Cross-Sectional Study. Cureus. 2020;12:e11615.
- [CrossRef] [Google Scholar]
- The Prevalence and Factors Associated with Obesity and Hypertension in University Academic Staff: A Cross-Sectional Study in Bangladesh. Sci Rep. 2023;13:7309.
- [CrossRef] [Google Scholar]
- Association of Different Obesity Patterns with Hypertension in US Male Adults: A Cross-Sectional Study. Sci Rep. 2023;13:10551.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Waist Circumference and the Prevalence of (Pre) Hypertension among 27,894 US Adults. Front Cardiovasc Med. 2021;8:717257.
- [CrossRef] [PubMed] [Google Scholar]
- Waist Circumference Change is Associated with Blood Pressure Change Independent of BMI Change. Obesity (Silver Spring). 2020;28:146-53.
- [CrossRef] [PubMed] [Google Scholar]
- The Characteristics of Elevated Blood Pressure in Abdominal Obesity Correspond to Primary Hypertension: A Cross-Sectional Study. Br Med Cardiovasc Disord. 2023;23:161.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting Transforming Growth Factor-β Receptors in Pulmonary Hypertension. Eur Respir J. 2021;57:2002341.
- [CrossRef] [PubMed] [Google Scholar]
- TGF-β: The Missing Link in Obesity-Associated Airway Diseases? Curr Res Pharmacol Drug Discov. 2021;2:100016.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic Approaches for Treating Pulmonary Arterial Hypertension by Correcting Imbalanced TGF-β Superfamily Signaling. Front Med (Lausanne). 2022;8:814222.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Inflammatory Chemokines in Hypertension. Pharmacol Ther. 2021;223:107799.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Inflammation, Immunity, and Oxidative Stress in Hypertension: New Insights and Potential Therapeutic Targets. Front Immunol. 2023;13:1098725.
- [CrossRef] [PubMed] [Google Scholar]
- The Link between Immunity and Hypertension in the Kidney and Heart. Front Cardiovasc Med. 2023;10:1129384.
- [CrossRef] [PubMed] [Google Scholar]
- The Immunomodulatory Effects of Antihypertensive Therapy: A Review. Biomed Pharmacother. 2022;153:113287.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int J Mol Sci. 2019;20:3458.
- [CrossRef] [PubMed] [Google Scholar]
- Immunomodulatory Activity of the Most Commonly Used Antihypertensive Drugs-Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers. Int J Mol Sci. 2022;23:1772.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and Change of miRs 145, 221 and 222 in Hypertensive Subjects Treated with Enalapril, Losartan or Olmesartan. Biomedicines. 2021;9:860.
- [CrossRef] [PubMed] [Google Scholar]
- Real-World Effectiveness and Safety of a Single-Pill Combination of Olmesartan/Amlodipine/Hydrochlorothiazide in Korean Patients with Hypertension and Cardiovascular Risk Factors. Adv Ther. 2023;40:4817-35.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammaging Markers Characteristic of Advanced Age Show Similar Levels with Frailty and Dependency. Sci Rep. 2021;11:4358.
- [CrossRef] [PubMed] [Google Scholar]
- A Cross-Sectional Study to Correlate Antioxidant Enzymes, Oxidative Stress and Inflammation with Prevalence of Hypertension. Life Sci. 2023;313:121134.
- [CrossRef] [PubMed] [Google Scholar]








