Translate this page into:
Arterial Pulse
Jyotsna Maddury, MD, DM, FACC, FESC, FICC Department of Cardiology Nizam's Institute of Medical Sciences, Punjagutta, Hyderabad, Telangana, 500082 India mail2jyotsna@rediffmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Introduction
The arterial pulse is an important vital sign. It is the abrupt expansion of an artery resulting from the sudden ejection of blood into the aorta and its transmission throughout the arterial system. Ejection of blood with every cardiac contraction is converted to flow, pressure, and dimension pulsations in arteries throughout the body. Although the term “pulse” refers to any such pulsation, the arterial pulse perceived by a clinician is the pressure pulse in a large, accessible artery. The impulse that results from left ventricular (LV) ejection can be transmitted down the aorta at a velocity 20 times greater than the velocity of the ejected blood bolus. The peak of this arterial pulse is the systolic blood pressure.1
History
In ancient times, palpation of the pulse was practiced in Egypt, China, and India. Until the 17th century, the clinical examination consisted of palpating the pulse and inspecting the urine to reveal disease and predict prognosis. In Chinese acupuncture, the pulse was timed according to the physician's respiration whereas digital pressure was applied to elicit information. Galen wrote 18 books on the arterial pulse in the second century, providing elaborate descriptions that influenced clinical practice well into the 18th century. The 1-minute pulse watch, invented by Floyer in 1707, offered the first opportunity to measure the heart rate accurately; however, this did not become a routine part of medical practice until the mid-19th century. Since the 19th-century observations by Dominic Corrigan (1832), the carotid arterial pulse has been linked to aortic valve disease and is essential for timing systole at the bedside. Kymographic recording of pulses (1847), polygraphic recording of pulses (1883), Marey's pulse writer—the sphygmograph—was used for recording the external pulsation of the heart and arteries and was a prototype for noninvasive devices in cardiology. In 1847, Carl Ludwig in Leipzig invented the kymograph, a pulse writer that would elevate physiology to a new level and be used to inscribe arterial and venous pulses. Pulsus alternans was described by Ludwig Traube in 1872, and Adolf Kussmaul called attention to the paradoxical pulse in 1873, noting that the arterial pulse could transiently disappear on inspiration even though the heart sounds were still audible. Before electrocardiography, in 1902, arterial pulse recordings were applied to diagnose arrhythmias, as shown by James Mackenzie in “The Study of the Pulse.”2
Technique
Pulses should be palpated when the patient is in a reclining or resting position. The index finger or thumb can lightly compress the artery during auscultation of the heart. Heart sounds can then be used as reference points for systole and diastole. An attempt to create a mental image of the arterial pulse, its ascending limb, peak, and descending limb enhances the value of palpation. Palpation of the opposite radial artery, while observing the pulse contour on the scope, can enhance palpation skills. Deep palpation may be necessary for subclavian, popliteal, or femoral pulses, particularly in obese patients. Flexing the knee and holding it while palpating the popliteal space perpendicular to the artery help in examination of the popliteal artery.
Basic Science
It is opposed by several forces that impede the development of flow and are interrelated in a complex manner. Three major determinants of arterial impedance include resistance, inertia, and compliance. Resistance is related to blood viscosity and the geometry of the vasculature; it opposes flow and is unaffected by changes in heart rate. Inertia, which is related to the mass of the column of blood, opposes the rate of change of arterial blood flow (i.e., acceleration) and depends on the heart rate. Compliance is related to the distensibility of the vascular walls, opposes changes in arterial blood volume, and depends on heart rate. Heart rate dependency of inertia and compliance introduces phase shifts between instantaneous pressure and flow in a pulsatile system. Inertia and compliance are important determinants of the character of ventricular ejection, especially in early systole, when flows and pressures change rapidly. The rapid-rising portion of the arterial pressure curve is often termed the “anacrotic limb” (from the Greek meaning upbeat). In experimental animals and in humans, peak proximal aortic flow velocity occurs slightly earlier than peak pressure. After its peak, aortic pressure declines as LV ejection slows, and peripheral blood flow continues. During isovolumic relaxation, a transient reversal of flow from the central arteries toward the ventricle just before aortic valve closure is associated with an incisura on the descending limb of the aortic pressure pulse. The subsequent smaller, secondary positive wave has been attributed to the elastic recoil of the aorta and aortic valve but is partially caused by reflected waves from more distal arteries. Subsequently, aortic pressure decreases again as further runoff in the peripheral circulation occurs in diastole.
In contrast, conventionally, the pulse is described in the time domain, where it is considered as a change in arterial pressure with time. An alternative, quantitative approach is to analyze the pulse in the frequency domain. Pulse is conceived as a composite wave that can be resolved into component harmonics similar to a musical wave. Impedance is the measure of the opposition to flow presented by a system and can be measured when harmonic analysis is used to relate frequency components of pressure and flow pulses. Usually there is a linear relation between pressure and flow at the same point in an artery and between pressures at different points in the arterial system. From impedance curves, it is possible to identify the factors responsible for the relationship between the pulsatile pressure and flow. The peripheral arterial pressure wave recorded is the summation of the incident (initial) and reflected waves. The systemic circulation has been represented by a simple asymmetric T-tube model that emphasizes the importance of wave reflection at two arteriolar reflecting sites in the upper and lower parts of the body. An important patient study indicates major reflection sites at the aortic level of the renal arteries and at a point distal to the terminal abdominal aorta bifurcation.
The arterial pulse changes as it travels from the central aorta down to the peripheral arteries. Although mean blood pressure decreases from the central aorta to the peripheral arteries, the systolic pressure increases.
The pulse can be distorted and damped by
-
Reflected, resonance, or standing waves
-
Differences in the elastic properties and caliber of the peripheral arteries of the upper and lower parts of the body.
The size of the normal arterial pulse is affected by
-
The LV ejection volume and the rate of ejection
-
The elasticity or distensibility of the peripheral arteries.
The greater the distance from the heart to a peripheral artery, the greater will be the distortion of the arterial pulse peripherally. Because complex factors affect the contour of the arterial pulse as it is transmitted through the arterial system, careful examination of the most central arterial pulse, the carotid, is necessary for pulse contour information. In the distal arteries, the peak becomes sharper, the dicrotic notch becomes more obvious, and pulse wave amplification occurs.3 Both the peak systolic and pulse pressures are amplified with increasing distance from the aortic valve. Interestingly, although the peak systolic pressure and pulse pressures are higher in the descending aorta than in the ascending aorta, the mean pressures are the same.4 Occurrence of the reflected wave at diastole is highly desirable because augmentation of pressure during diastole aids coronary perfusion (Fig. 1).

-
Fig. 1 Changes in the arterial pressure from center to periphery. The pulse pressure and systolic amplitude increase, and the ascending limb of the pulse wave becomes steeper. The incisura of the central aorta pulse is gradually replaced by a smoother, somewhat later dicrotic notch that occurs at lower pressure levels.
Fig. 1 Changes in the arterial pressure from center to periphery. The pulse pressure and systolic amplitude increase, and the ascending limb of the pulse wave becomes steeper. The incisura of the central aorta pulse is gradually replaced by a smoother, somewhat later dicrotic notch that occurs at lower pressure levels.
Peripheral transmission of the arterial pulse: As the normal aortic pulse wave is transmitted peripherally, significant changes in its contour occur caused by
-
Distortion and damping of pulse wave components
-
Different rates of transmission of various components
-
Distortion or exaggeration by reflected, resonant, or standing waves
-
Conversion of kinetic energy into hydrostatic or potential energy
-
Differences in distensibility and caliber of the arteries
-
Changes in the vessel wall caused by age, disease, or both
Transformation of the ventricular pressure pulse, with its intermittent flow and large pressure changes, into the peripheral pulse, with its continuous flow and smaller pressure changes, is caused by the initial transfer of kinetic to potential energy in the aorta in systole and subsequent reclamation of this stored energy in diastole.
The arterial pulse is altered by several factors
-
Heart rate (increased diastolic pressure with increased heart rate).
-
Stroke volume (systolic and pulse pressure increase with increased stroke volume).
-
Aortic valve function (increased pulse pressure and decreased diastolic pressure with aortic insufficiency, decreased pulse pressure and a slow rate of increase in pressure with aortic stenosis [AS]).
-
Arterial compliance (increased pulse pressure and peaked waveform with decreased compliance) and transmission of the pressure wave through the arterial circulation. The latter results from waves reflected at branch points, changes in arterial compliance, and differential transmission between the high- and low-frequency components of the arterial pressure waveform.5
The arterial pressure pulse enters the proximal aorta and travels distally at a velocity many times faster than that of maximum blood flow. The pressure wave is accompanied by a traveling wave distending the arterial wall with the pulse wave velocity increasing as arterial wall distensibility diminishes.
The pulse wave arrives progressively later at more peripheral sites when timed from the QRS complex on the electrocardiogram (ECG). Representative time delays from the central aorta are as follows: carotid, 30 milliseconds; brachial, 60 milliseconds; radial, 80 milliseconds; and femoral, 75 milliseconds.
Although the carotid pulse is important, there is much to be learned from an examination of all pulses bilaterally: subclavian, brachial, femoral, popliteal, posterior tibial, and dorsalis pedis. Palpation of the peripheral arteries can be performed whenever they are close enough to the skin surface to be compressed. Auscultation of these arteries, especially the carotid, subclavian, and femoral, can reveal bruits suggesting partial obstruction. The pulse rate, rhythm, and quality can be examined. In addition, the patency and pliability of the artery can be assessed.6
Examination of Pulse
During pulse examination, we have to see
-
Pulse rate.
-
Regularity of pulse.
-
Quality of the pulse.
-
The thickness and hardness of the arterial walls often can be assessed by rolling the vessel against underlying tissue
Pulse rate changes with the patient's age. A fast pulse rate for age may indicate arrhythmia or congestive heart failure (CHF). A slow pulse rate usually reflects athletic conditioning; however, atrioventricular block or drug effect should be considered. Irregularity of pulse rate may indicate an arrhythmia. However, a change in pulse rate with respiration is normal (sinus arrhythmia).7
Clinical Significance
Assessing the Pulses
The examiner uses tactile receptors in the tips of the fingers to sense movement of the arterial wall associated with the pressure pulse as it passes the site of palpation. Measurements in the proximal aorta show cyclic movement in both diameter and length proportional to the pulse pressure. In more peripheral arteries with connective tissue attachments, however, the detectable movement is small and variable, with radial expansion by only approximately 2% of the end-diastolic cross-sectional area.
The usual technique for palpating the arterial pulse is to press with the examining fingers until the maximum pulse is sensed. The pulse is felt as changing displacement superimposed on the baseline displacement produced by compressing the artery. The examiner should apply varying degrees of pressure while concentrating on the separate phases of the pulse wave. This method, referred to as trisection, is useful for assessing the upstroke, systolic peak, and diastolic slope of the arterial pulse.
Readily palpable pulses in healthy individuals include the brachial, radial, and ulnar arteries of the upper extremities and the femoral, popliteal, dorsalis pedis, and posterior tibial arteries of the lower extremities. The aorta also can be palpated in thin people.
The carotid pulse is usually best examined with the sternocleidomastoid muscles relaxed and the head rotated slightly toward the examiner. The carotid pulse can be timed from the first heart sound, which is heard slightly before the pulsation. The carotid pulse should be palpated in the lower half of the patient's neck to avoid carotid sinus compression.
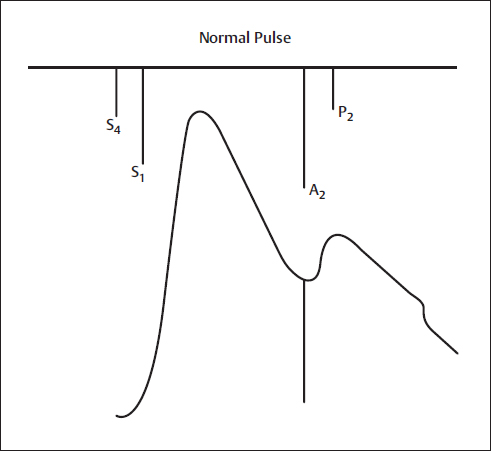
Normally, the incident (or percussion wave) begins with systolic ejection (just after S1) and is the predominant monophasic pulse appreciated at the bedside. The incisura or dicrotic notch signifies aortic valve closure (Fig. 2).
-
Fig. 2 Normal pulse. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Fig. 2 Normal pulse. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
The carotid artery pulse wave occurs within 40 milliseconds of the ascending aortic pulse and reflects aortic valve and ascending aortic function. The aortic pulse is best appreciated in the epigastrium (abdominal aorta). The temporal arteries can be easily palpated and aid in the diagnosis of temporal arteritis or polymyalgia rheumatica. One of the two pedal pulses may not be palpable in a normal patient because of unusual anatomy (posterior tibial, < 5%; dorsal pedis, < 10%), but each pair should be symmetric. True congenital absence of a pulse is rare (< 2%), and in most cases, pulses can be obtained with a handheld Doppler device when not palpable.
The grading of the pulse are:
-
Complete absence of pulsation
-
Small or reduced pulsation
-
Normal or average pulsation
-
Large or bounding pulsation
Simultaneous Palpation of Pulse in the Upper and Lower Extremities
Brachial pulses should be palpated simultaneously as well.7
-
If there is a delay between the upper and lower extremity pulses or absence of femoral pulsations, coarctation of the aorta should be considered.
-
Absent or weak pulses in the arm may result from
-
Previous subclavian flap repair of a coarctation of the aorta
-
Systemic-to-pulmonary artery shunts (i.e., classic Blalock-Taussig shunt)
-
-
Bounding pulses reflect aortic runoff as in aortic regurgitation (AR), patent ductus arteriosus (PDA), or hyperkinetic states such as fever, anemia, and thyrotoxicosis, or in pathologic states such as severe bradycardia, AR, arteriovenous fistula (AVF) or arteriovenous malformations (AVMs).
-
Diminished pulses are ominous in children and may suggest cardiac failure or shock.
The contour of the pulses depends on
-
The stroke volume
-
Ejection velocity
-
Vascular capacity and compliance
-
Systemic resistance
The palpable pulse reflects the merging of both the ante-grade pulsatile flow of blood and reflection of the propagated pulse returning from the peripheral arteries. The arterial pulse upstroke increases with distance from the heart.
Normal Contour of Pulse (Normal Arterial Pulse)
The normal carotid pulse has a smooth, rapid upstroke or ascending limb to a smooth, dome-shaped summit. Then a downstroke occurs that is somewhat less rapid than the upstroke. The dicrotic notch and secondary diastolic wave are usually not felt but can be palpable in some normal individuals, particularly during fever, exercise, or excitement. The dicrotic notch usually occurs approximately 300 milliseconds after the onset of the pulse wave when corrected for heart rate.
Graphic recordings of the arterial pulses frequently show two positive deflections during systole, the first shoulder being referred to as the percussion wave and the second as the tidal wave. In the normal proximal aortic pulse, the percussion wave is caused by arrival of the impulse generated by LV ejection, the tidal wave can represent its echo from the upper part of the body, and the dicrotic or diastolic wave is a reflection from the lower part of the body.
Amplitude of Pulse
Because peripheral pulse contour is determined by LV ejection and vascular properties, emphasis is placed on the presence or absence of a pulse and on hypokinetic or hyperkinetic pulses.
Hypokinetic Pulse
If the pulse is present but of low volume and amplitude, it is hypokinetic. This could suggest low cardiac output in shock or myocardial infarction. Idiopathic dilated cardiomyopathy, valvular stenosis, pericardial tamponade, or constrictive pericarditis can also cause low cardiac output and small peripheral pulses. Severe AS causes a small pulse known as pulsus parvus et tardus. It is a low-amplitude pulse with a delayed upstroke. This is best palpated on the carotid artery.
Hyperkinetic Pulse
If the pulses become bounding, they are called hyperkinetic. Anxiety, exercise, fever, hyperthyroidism, and anemia can cause a hyperkinetic pulse in a normal person with a large LV stroke volume and an otherwise normal cardiovascular system. Hyperkinetic pulses can also occur where there is a rapid peripheral runoff of blood in addition to a large stroke volume from the left ventricle (LV). PDA with normal pulmonary pressures, large arterial venous fistulas, and severe AR can cause these hyperkinetic pulses. The pulse of severe AR is described as water-hammer and collapsing. Severe AR can also cause a pulse in the fingernail bed (Quincke's pulse), best demonstrated by placing a penlight on the finger pad and casting light through the fingernail from behind. Varying the pressure of the penlight on the finger pad will bring out the Quincke's pulse. A slow, bounding hyperkinetic pulse can be found in complete heart block.
Regular Pulse
Bisferiens Pulse
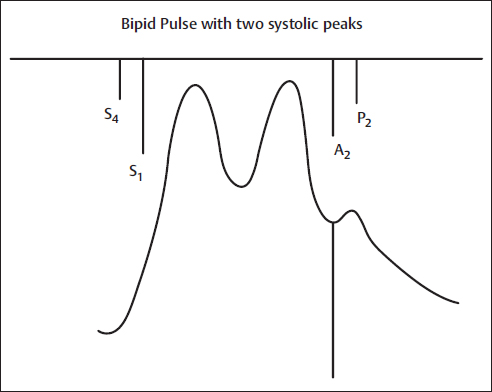
The bisferiens (from the Latin twice beating) pulse has a waveform characterized by two positive waves during systole. The pulse wave upstroke rises rapidly and forcefully, producing the first systolic peak (percussion wave). A brief decline in pressure is followed by a smaller and somewhat slower-rising positive pulse wave (tidal wave). Abnormalities of LV ejection and reflected waves from peripheral arteries contribute to the prominence of the second systolic wave in the bisferiens pulse. The bisferiens pulse is sometimes more easily palpable in a brachial or radial artery. The bisferiens pulse can be elicited by maneuvers that decrease the LV size or increase its contractility (Figs. 3, 4).
-
Fig. 3 Bifid pulse with two systolic peaks. Example is severe aortic regurgitation. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Fig. 3 Bifid pulse with two systolic peaks. Example is severe aortic regurgitation. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
-
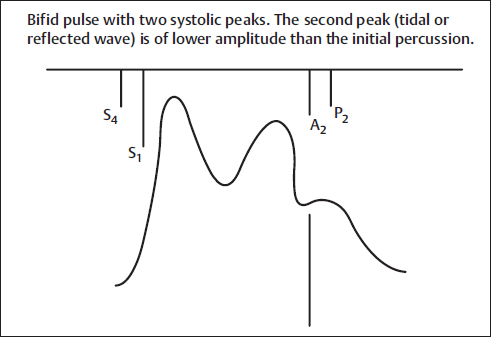
Fig. 4 Bifid pulse with two systolic peaks. The second peak (tidal or reflected wave) is of lower amplitude than the initial percussion wave. Example is hypertrophic obstructive cardiomyopathy (HOCM). A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Fig. 4 Bifid pulse with two systolic peaks. The second peak (tidal or reflected wave) is of lower amplitude than the initial percussion wave. Example is hypertrophic obstructive cardiomyopathy (HOCM). A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Bisferiens pulse occurs
-
In patients with pure AR.
-
When some AS accompanies the severe AR.
-
Hypertrophic cardiomyopathy (HCM): Many of whom have a pressure gradient in the LV outflow tract. The midsystolic negative wave usually coincides with a marked decrease in the rate of LV ejection. The second systolic wave, or tidal wave, is most likely produced by reflected waves from the periphery. A physical finding nearly specific for HCM is a much smaller arterial pressure pulse in the cardiac cycle following a premature ventricular beat.
Dicrotic Arterial Pulse
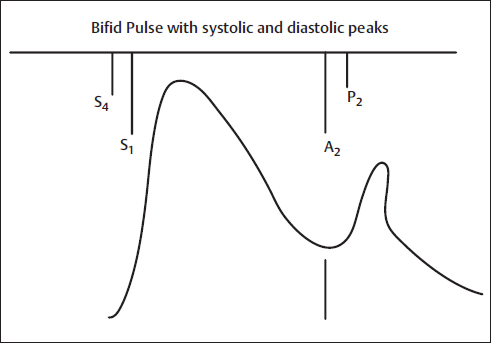
The dicrotic (from the Greek dikrotos, “double beating”) pulse is a twice-peaked pulse with one peak in systole and the second in diastole, the latter caused by an accentuated and palpable dicrotic wave that follows the second heart sound. It is usually best felt in the carotids, although it also can be palpated over more peripheral arteries. Major abnormalities include a short systolic ejection phase, a low dicrotic notch, a large diastolic wave, a narrow pulse pressure, a diminished rate of rise in the pulse, and the lack of distinct percussion and tidal waves. Low cardiac output states can allow the systolic and diastolic pulses to become nearly equal and potentially palpable (Fig. 5).
-
Fig. 5 Bifid pulse with systolic and diastolic peaks. Example is intra-aortic balloon counterpulsation. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Fig. 5 Bifid pulse with systolic and diastolic peaks. Example is intra-aortic balloon counterpulsation. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Causes of dicrotic pulse are
-
Low cardiac output states
-
Patient with fever or after exercise in a normal individual and is consistent with increased vascular compliance
-
Pulse is intra-aortic balloon counterpulsation
Pulsus Parvus Et Tardus
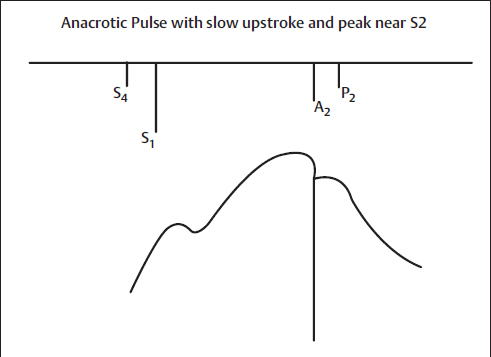
Severe AS may be suggested by an arterial pulse that is small and has a delayed systolic peak. Occasionally, there can be a detectable shoulder on the upstroke of the carotid pulse, referred to as anacrotic. Palpable coarse vibrations are often present as a systolic thrill over the slowly rising carotid pulse. The parvus et tardus pulse is much easier to detect in the carotid arteries than in more distal arteries (Fig. 6).
-
Fig. 6 Anacrotic pulse with slow upstroke and peak near S2. Example is aortic stenosis. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Fig. 6 Anacrotic pulse with slow upstroke and peak near S2. Example is aortic stenosis. A2, aortic component of S2; P2, pulmonic component of S2; S1, first heart sound; S2, second heart sound; S4, fourth heart sound.
Most middle-aged patients with uncomplicated severe AS have a parvus et tardus pulse, but this pulse can also occur in relatively mild stenosis. Conversely, an apparently normal arterial pulse is not unusual in elderly patients with severe AS who have decreased distensibility of the large arteries. Severe LV failure often results in a small, weak pulse that can be difficult to distinguish from that of AS. Carotid-apical delay is assessed during simultaneous auscultation of the heart sounds; the carotid upstroke should be coincident with S1. This finding is less accurate in older hypertensive patients with reduced vascular compliance and stiffer carotid arteries.
Corrigan or Water Hammer Pulse
An abrupt carotid upstroke with rapid falloff characterizes the pulse of AR. The carotid upstroke is also rapid in older patients with isolated systolic hypertension and wide pulse pressures.
Pulsus Alternans
Pulsus alternans is defined by the beat-to-beat variability of the pulse amplitude. It is present when only every other phase 1 Korotkoff sound is audible as the cuff pressure is slowly lowered, in a patient with a regular heart rhythm, independent of the respiratory cycle. These amplitude changes will be emphasized in a peripheral artery and may be easiest to detect in the femoral pulse, which normally has a slightly wider pulse pressure than the carotid artery. The patient's respiration should be held because the small changes in arterial pressure caused by normal respiration can obscure the recognition of pulsus alternans. Pulsus alternans can be confirmed by using a sphygmomanometer and is usually associated with an LV third heart sound.
In pulsus alternans, beats occur at regular intervals with a regular alternation of the systolic height of the pressure pulses. Rarely, pulsus alternans is so marked that the weaker pulses are not felt at all. When pulsus alternans is noticed first after a premature beat, the extent of the difference in systolic pressure in alternating beats can decline for several cycles until the pulse amplitude is again constant. The initiation of post-premature ventricular beat pulsus alternans is probably related to the increased duration of LV filling after the premature beat.
Sustained pulsus alternans is seen in severe depression of LV performance. Sustained pulsus alternans is likely caused by alteration of the contractile state of at least part of the myocardium, which can be caused by the failure of electromechanical coupling in some cells during the weaker contraction. A subsequent stronger contraction would then represent contraction of all cells, some of which were potentiated (Fig. 7).

-
Fig. 7 Pulsus alternans.
Fig. 7 Pulsus alternans.
Pulsus alternans is generally seen in
-
Pulsus alternans is generally seen in severe heart failure.
-
It is exaggerated in severe AR, hypertension, and hypovolemic states.
-
It can also occur for several beats after supraventricular tachycardia in normal persons or when the respiratory rate is half the pulse rate.
It is attributed to cyclic changes in intracellular calcium levels and action potential duration. Association with electrocardiographic T-wave alternans appears to increase arrhythmic risk.
Pulse Paradoxus
If the pulse decreases dramatically in amplitude with inspiration and increases with expiration, it is called pulsus paradoxus. Actually this is an exaggeration of a normal physiologic phenomenon. On inspiration, there is some pooling of blood in the lungs and a transient decline in venous return to the LV. Therefore, LV stroke volume declines slightly. If the fall in systolic blood pressure is greater than 10 mm Hg, it is abnormal. Pulsus paradoxus is measured by noting the difference between the systolic pressure at which the Korotkoff sounds are first heard (during expiration) and the systolic pressure at which the Korotkoff sounds are heard with each beat, independent of respiratory phase. Between these two pressures, the sounds are heard only intermittently (during expiration). The cuff pressure must be decreased slowly to appreciate the finding. Tachycardia, atrial fibrillation (AF), and tachypnea make its assessment difficult. Pulsus paradoxus may be palpable when the pressure difference exceeds 15 to 20 mm Hg.
In patients with cardiac tamponade, fluid accumulation in the pericardium increases intrapericardial pressure, and the heart's filling capacity is reduced. During inspiration, the expected augmentation of venous return to the right side of the heart occurs despite the elevated intrapericardial pressure. This produces a leftward shift in the interventricular septum that reduces LV end-diastolic volume. This produces a decline in LV stroke volume and systolic blood pressure during inspiration. Pulsus paradoxus is common with cardiac tamponade but infrequent with constrictive pericarditis. Different hemodynamic mechanisms contribute to the production of a paradoxical pulse in certain patients with superior vena cava obstruction, asthma, or obstructive airways disease.8
Causes of pulsus paradoxus are
-
Pericardial tamponade
-
Constrictive pericarditis
-
Superior vena cava obstruction
-
Asthma or obstructive airways disease
-
Pulmonary embolism or shock
-
Patients after thoracotomy
-
In obesity and pregnancy without clinical disease
-
Hemorrhagic shock
-
Tension pneumothorax
Absent Pulses
A pulse in the foot should not be considered absent unless examined with the foot in a dependent position. Absence of a pulse could suggest occlusion by thrombus, embolus, or dissection. Unilateral absence of a pulse can aid in the diagnosis of a dissected aortic aneurysm. History and physical examination findings can help assess the level of arterial obstruction in lower extremity claudication. Auscultation for aortic and femoral artery bruits should be routine. The correlation between the presence of a bruit and the degree of vascular obstruction is weak. A cervical bruit is a poor indicator of the degree of carotid artery narrowing, and the absence of a bruit does not exclude significant luminal compromise. Extension of a bruit into diastole or a thrill generally indicates severe stenosis. Other causes of a bruit include AVFs and enhanced flow through normal arteries as, for example, in a young patient with fever. Abnormal pulse oximetry, defined by a more than 2% difference between finger and toe oxygen saturation, can also indicate lower extremity peripheral arterial disease (PAD) and is comparable to the ankle brachial index (ABI) (likelihood ratio [LR], 30; 95% confidence interval [CI]: 7.6–121.0 vs. LR, 24.8; 95% CI: 6.2–99.8).9
Preferred pulses to see the different types of pulses:
-
The carotid pulse for pulse contour
-
The femoral pulse for pulses paradoxus
-
The radial pulse for rate and rhythm
Carotid Pulse
Palpation of the carotid arterial pulse provides a clue to the LV stroke volume; a small pulse suggests a reduced stroke volume, whereas a sharp brief upstroke is often observed in patients with mitral regurgitation (MR) or ruptured ventricular septum with a left-to-right shunt. Pulsus alternans reflects severe LV dysfunction.8
Irregular Pulse
An irregular pulse can be caused by
-
AF
-
Premature beats arising in the atria, atrioventricular junction, or ventricles
-
Second-degree atrioventricular block
Premature complexes are among the most common causes of an irregular pulse.10 AF causes an irregularly, irregular pulse. Not only will the rate of the pulse be irregular, but the pulse amplitude will also vary. This results from variable stroke volumes during systole. Short R-R intervals during AF do not allow adequate time for LV diastolic filling, resulting in a low stroke volume and the absence of palpable peripheral pulse. This results in a “pulse deficit,” during which the peripheral pulse is not as rapid as the apical rate.10 Post extrasystolic potentiation contributes to the beat-to-beat variability of the pulse in AF. Simultaneous auscultation of the heart and palpation of the radial pulse can allow measurements of an apical to radial pulse deficit. Controlled AF should have no pulse deficit; that is, all central heartbeats are transmitted to the radial pulse and the peripheral circulation. Premature beats of any origin can cause irregularities of the pulse. If the premature beats alternate with normal sinus beats, bigeminy results. The results can be a pulse beat that has a strong, large-amplitude pulse (hyperkinetic) alternating with a weak, low-amplitude pulse (hypokinetic). Irregularities of the pulse rate or rhythm require an ECG for final diagnosis of the cardiac arrhythmia.11 12
Pulse in Different Diseases
Congenital Heart Disease
Patent Ductus Arteriosus during Neonatal Stage
-
No or minimal lung disease: Pulse pressure widens, and the peripheral pulses become more prominent and bounding as the left-to-right shunt increases. Increased peripheral pulses are best appreciated by the presence of palmar or forearm pulses.13
-
PDA in infants recovering from lung disease: Increasing precordial activity is a good clinical indication of the magnitude of shunting in these infants, and increased heart rate, pulse pressure, and bounding pulses with a rapid upstroke are often detectable early. Palmar or forearm pulses are often palpable. Because most of these infants have indwelling umbilical arterial catheters, careful monitoring of the umbilical arterial blood pressure often shows a widening pulse pressure and a decrease in diastolic pressure as left-to-right shunting develops.14
-
PDA associated with lung disease: Changes in the ventilatory status may occur due to progression of the primary pulmonary disease, and it is often even more difficult to separate LV failure from increasing pulmonary problems than in the previous group. Increasing precordial activity, bounding pulses, and a widening arterial pulse pressure suggest the development of left-to-right shunting. When present, the murmur is usually only systolic, the pulmonic component of the second sound is accentuated, and a gallop rhythm is often heard.14
-
PDA with severe LV failure: The pulse volume decreases.15
-
PDA with pulmonary arterial hypertension (PAH): Pulses become less bounding.
-
PDA with Eisenmengerization: The pulses are either of normal or small volume.16
The Closing Ductus Physiology Like in Hypoplastic Left-Sided Herat Syndrome
A sudden deterioration takes place with rapidly progressive congestive failure and shock as the ductus arteriosus constricts. There are decreased systemic blood flow (SBF) and increased pulmonary blood flow (PBF), which are largely independent of the pulmonary vascular resistance (PVR). The peripheral pulses are weak to absent.17
Coarctation of Aorta
The hallmark physical findings in coarctation consist of discrepant arterial pulses and systolic blood pressures in the upper and lower extremities. Arterial pulses below the coarctation are diminished in amplitude and delayed in timing compared with the proximal pulses. Systolic blood pressure is elevated proximal to the coarctation, and a systolic pressure gradient is present between the arm and leg.
Several clinical circumstances may make detection of pulse and pressure discrepancies difficult.
-
The coarctation pressure gradient may be minimal, sometimes as a result of a mild coarctation, but also in an infant with heart failure and diminished cardiac output or with a large PDA. Descending aorta flow may be maintained by a right-to-left ductal shunt, and in the presence of a large ventricular septal defect, the perfusion may be well oxygenated and pulsatile.
-
Detection of arterial pulse and pressure differences may be difficult because of variations in brachiocephalic artery anatomy.
-
An anomalous right subclavian artery arises distal to the coarctation in approximately 3 to 4% of cases. In these patients, the arterial pulse and blood pressure are identical in the right arm and leg, and discrepancies are detected only in the left arm.
-
In other patients, the left subclavian artery arises adjacent to the coarctation, and its orifice may be stenotic. In such patients, a bounding arterial pulse and elevated systolic pressure will be detected only in the right arm.
-
Rarely, patients may present with an anomalous right subclavian artery and a stenotic left subclavian artery. In these patients, arterial differences in the four extremities will not be detected, although carotid artery pulsations will be bounding.18
-
-
Associated significant AR coexists.
Aortic Arch Interruption
Absent upper and lower limb pulses, with a palpable superficial temporal pulse, are a helpful clue to the diagnosis.19
Hypoplastic Left-Sided Heart Syndrome
The upper and lower extremity pulses are palpable and symmetric early but are reduced later as ductal closure ensues.20
Tetralogy of Fallot
Arterial pulses will be normal in uncomplicated tetralogy of Fallot (TOF).
The pulse pressure may be widened reflecting arterial diastolic runoff in the presence of21
-
Aortopulmonary collaterals
-
Palliative surgical shunt
-
PDA
Tricuspid Atresia
Peripheral pulses are normal except when associated with coarctation of the aorta.
Uhl's Anomaly
In Uhl's anomaly, at physical examination, along with cyanosis, hepatomegaly, jugular venous distension with a dominant a wave, the quiet precordium, and peripheral pulses often are diminished in amplitude.22
Left Ventricular Inflow Obstruction
Decreased pulse volume due to congestive cardiac failure (CCF).
Pulmonary Atresia with Intact Ventricular Septal Defect
The peripheral pulses and blood pressure usually are normal in the neonatal period, even with a PDA, because the runoff into the lungs is not excessive during the first few weeks of life. In cyanotic patients, the pulses are normal. However, in patients beyond the first 4 to 6 weeks of age in whom PBF through a PDA, collaterals, or surgically created shunt is substantial, the pulses are bounding and only minimal cyanosis may be present.23
Truncus Arteriosus
The peripheral pulses are accentuated and may be bounding if truncal valve regurgitation is there, if not then normal pulse. The pulse pressure usually is increased owing to runoff into the pulmonary vascular bed during diastole.24
Transposition of the Great Arteries
A large, persistent PDA can modify the clinical findings in the neonate with transposition of the great arteries/intact ventricular septum (TGA/IVS) because a large intercirculatory shunt may be present. Characteristically, these infants present quite early with prominent tachypnea and relatively slight cyanosis. Importantly, the classic signs indicative of a large PDA, such as continuous murmur, bounding pulses, and a prominent mid-diastolic rumble, are present in fewer than half of this group.25
Congenital Anomalies of the Aortic Root
Aortic-LV defect (tunnel) and aneurysm of sinus of Valsalva: Many of patients with aortic-LV defect present in infancy with CHF. They have signs resembling marked aortic incompetence—a wide pulse pressure with a low diastolic blood pressure, a hyperactive dilated LV and enlarged left atrium, and a loud to-and-fro murmur at the base.26
Aortic Arch Anomalies
Physical findings of pulse discrepancy, depending on branching pattern, are helpful only after restoration of satisfactory cardiac output. Absence of all limb pulses suggests interruption type B with anomalous subclavian artery, that is, both carotid arteries proximal and both subclavian arteries distal to the interruption. Strong carotid pulses help differentiate interrupted arch from critical AS in which all pulses are diminished.27
Left aortic arch with isolated subclavian artery: In many cases there may be no symptoms or simply absence of the right arm pulse.
Isolated brachiocephalic arteries: Cases of isolated brachiocephalic arteries may have diminished pulses or lower blood pressure in the affected artery. If the ductus remains patent, pulmonary artery steal can occur with flow down the vertebral artery through the ductus into the low-resistance pulmonary artery. The diagnosis should be suspected in any patient with right aortic arch and diminished pulse amplitude or blood pressure in the left arm.28
Cervical arches: In the presence of a pulsatile neck mass, a presumptive diagnosis can be made by notation of loss of femoral pulses during brief compression of the mass.29
Valvular Heart Diseases
Mitral stenosis
The arterial pulse is usually normal, but in patients with a reduced stroke volume, the pulse may be low in volume.
Mitral Regurgitation
Brisk arterial pulses are frequently present. Palpation of the arterial pulse is helpful in differentiating AS from MR, both of which may produce a prominent systolic murmur at the base of the heart and apex. The carotid arterial upstroke is sharp in severe MR and delayed in AS; the volume of the pulse may be normal or reduced in the presence of heart failure.30
Acute Severe Aortic Regurgitation
Patients with acute severe AR are tachycardic and tachypneic. Unlike the clinical examination found with significant chronic AR, the pulse pressure is often narrow and the pulses are not increased or bounding.31
Chronic Severe Aortic Regurgitation
On examination, significant chronic AR results in a wide pulse pressure (elevated systolic and low diastolic pressures) and bounding pulses. The water-hammer pulse in severe AR is named after a Victorian toy. In patients with chronic, severe AR, the head may bob with each heartbeat (de Musset's sign), and there are water hammer pulses, with abrupt distention and quick collapse (Corrigan's pulse). The arterial pulse is often prominent and can be best appreciated by palpation of the radial artery with the patient's arm elevated. A bisferiens pulse may be present and is more readily recognized in the brachial and femoral arteries than in the carotid arteries. A variety of auscultatory findings provide confirmation of a wide pulse pressure. Traube's sign (a.k.a. pistol shot sounds) refers to booming systolic and diastolic sounds heard over the femoral artery, Müller's sign consists of systolic pulsations of the uvula, and Duroziez's sign consists of a systolic murmur heard over the femoral artery when it is compressed proximally and a diastolic murmur when it is compressed distally. Capillary pulsations (Quincke's sign) can be detected by transmitting a light through the patient's fingertips or exerting gentle pressure on the tip of a fingernail.30
Aortic Stenosis
With severe stenosis, the arterial pulse may be decreased, with a slow rate of rise. If there is associated AR, however, the pulses may be increased.
The carotid upstroke directly reflects the arterial pressure waveform. The expected finding with severe AS reveals a slow-rising, late-peaking, low-amplitude carotid pulse and the parvus and tardus carotid impulse. When present, this finding is specific for severe AS. However, many adults with AS have concurrent conditions, such as AR or systemic hypertension, that affect the arterial pressure curve and the carotid impulse. Thus, an apparently normal carotid impulse is not reliable for excluding the diagnosis of severe AS. With severe AS, radiation of the murmur to the carotids may result in a palpable thrill or carotid shudder.32
When the LV fails and stroke volume falls, the systolic murmur of AS becomes softer; rarely, it disappears altogether. The slow rise in the arterial pulse is more difficult to recognize. Stated simply, with LV failure, the clinical picture changes from typical AS to that of severe LV failure with a low cardiac output. Thus, occult AS may be a cause of intractable heart failure, and severe AS should be ruled out by echocardiography in patients with heart failure of unknown cause because operative treatment may be lifesaving and result in substantial clinical improvement.32
In more severe cases of neonatal AS, the left heart may not have the capacity to handle the entire cardiac output and closure of the ductus arteriosus may result in circulatory collapse. Such infants are pale and poorly perfused, with poor pulses, hepatomegaly, tachycardia, gallop rhythm, hyperdynamic right ventricular impulse, tachypnea with retractions, and usually but not always a systolic heart murmur.33
In supravalvular AS, the pulse in the right arm is frequently greater than that in the left arm because of Coanda's effect. This pulse disparity may relate to the tendency of a jet stream to adhere to a vessel wall (Coanda's effect) and selective streaming of blood into the innominate artery. Differences in upper extremity blood pressures and carotid artery pulses may also result from stenosis of the aortic arch branches.34
Cardiomyopathies
HCM: Arterial pulses usually rise rapidly with bisferiens contour. External carotid pulse recordings demonstrate a bifid pulse contour, shortened upstroke time, and increased systolic ejection period.35 36
The classic carotid pulsation is brisk with a spike and dome pattern, characterized by a rapid rise (percussion wave) followed by a midsystolic drop that is, in turn, followed by a secondary wave (tidal wave). The midsystolic drop in amplitude of the carotid pulse contour is caused by premature closure of the aortic valve and coincides with systolic anterior motion of the mitral valve. The late peak is due to relief of the outflow tract gradient as the mitral valve leaflet returns to its original position. In the presence of pronounced obstruction, there is a longer ejection time. The carotid pulsation with dynamic LV outflow tract obstruction differs from that of a fixed obstruction, such as seen with valvular or discrete subvalvular AS where there is a decrease in both the rate of rise and the amplitude of the pulsation.37 A low pulse volume, due to a reduced stroke volume and tachycardia, can be seen in severe cases.
Pericardial Diseases
Cardiac Tamponade
The characteristic features of cardiac tamponade include low cardiac output, elevated central venous pressures, a paradoxical pulse, muffled or diminished heart sounds, and tachycardia.38
The second characteristic hemodynamic finding is the paradoxical pulse. In severe tamponade, the arterial pulse is impalpable during inspiration.
The mechanism of the paradoxical pulse is multifactorial, but respiratory changes in systemic venous return are certainly important. In tamponade, in contrast to constriction, the normal inspiratory increase in systemic venous return is retained. Therefore, the normal decline in systemic venous pressure on inspiration is present (and Kussmaul's sign is absent). The increase in right-sided heart filling occurs, once again, under conditions in which total heart volume is fixed and left-sided heart volume is markedly reduced to start. The interventricular septum shifts to the left in exaggerated fashion on inspiration, encroaching on the LV such that its stroke volume and pressure generation are further reduced. Although the inspiratory increase in right-sided heart volume (preload) causes an increase in right ventricular stroke volume, this requires several cardiac cycles to increase LV filling and stroke volume and to counteract the septal shift. Other factors that may contribute to the paradoxical pulse include increased afterload caused by transmission of negative intrathoracic pressure to the aorta and traction on the pericardium caused by descent of the diaphragm, which increases pericardial pressure. Associated with these mechanisms are the striking findings that left- and right-sided heart pressure and stroke volume variations are exaggerated and 180 degrees out of phase.
Cardiac tamponade without paradoxical pulse occurs
-
When there are preexisting elevations in diastolic pressures or volume. Examples are patients with LV dysfunction, AR, and atrial septal defect.
-
In patients with retrograde bleeding into the pericardial sac due to aortic dissection; tamponade can occur without a paradoxical pulse because of aortic valve disruption and regurgitation.39
Constrictive Pericarditis
Paradoxical pulse occurs in perhaps one-third of patients with constriction, especially when there is an effusive-constrictive picture.
Hemopericardium
These patients may also have the combination of acute volume overload due to disruption of the aortic valve and tamponade without a paradoxical pulse.40
Miscellaneous
Vascular Anomalies
Systemic AVMs or AVFs usually are associated with wide pulse pressure and bounding pulses. In the new-born period, congenital AVMs and AVFs may clinically resemble congenital heart defects (CHDs), leading to a delay in diagnosis. Diminished femoral pulses may mimic aortic coarctation.41 In contrast, in pulmonary AVM the arterial pulse is normal.42
Aortic Dissection
Absent pulses depend on the site and extending of dissection. The physical findings most typically associated with aortic dissection—pulse deficits, AR, and neurologic manifestations— are more characteristic of ascending than descending dissection. Pulse deficit was reported in 19% of type A and only 9% of type B dissections. Aortic dissection may extend into the iliac arteries and cause diminished femoral pulses (12%) and acute lower extremity ischemia. The pulse deficits may be intermittent because the intimal flap movement sporadically obstructs the arterial orifice or because of distal reentry of blood into the true lumen, which decompresses the false channel.43
Atheroembolism
Blue toe syndrome with presence of pedal pulses is typically present because the emboli tend to lodge in the more distal digital arteries and arterioles.44
Takayasu
During the active inflammatory pre-pulseless phase, the peripheral pulses and blood pressures may be normal, making diagnosis difficult. As the disease progresses into the chronic pulseless phase, there are additional symptoms and signs of absent or decreased brachial and radial pulses or carotid pulses with bruits over the cervical, supraclavicular, and abdominal regions.45
Pulse in Heart Failure
Physical findings of heart failure are45
-
Tachycardia
-
Extra beats or irregular rhythm
-
Narrow pulse pressure or thready pulse
-
Pulsus alternans
Pulse in Hypertension and Arteriosclerosis
In hypertension and arteriosclerosis, the pressure pulse amplitude is increased, the tidal wave is prominent, and the diastolic wave is absent. All features of the pulse can be explained by increased wave velocity. Reflected waves return to the proximal aorta during late systole, augmenting the tidal wave and increasing systolic pressure.
Chronic Kidney Disease (CKD) on Hemodialysis
Patients on chronic hemodialysis often have hyperdynamic pulses produced by the combination of a surgical AVF, anemia, and hypertension.
Pulse after Cardiac Catheterization
After removal of arterial sheath, just enough pressure is needed to prevent bleeding from the vessel, not necessarily completely occluding all flow through the vessel. While holding pressure, the pedal pulses should be palpated often to ensure adequate perfusion.
After a diagnostic catheterization, pulse loss is rare. Even in small infants, the use of a 3F pigtail catheter tapered to a 0.021-in wire should allow a safe retrograde arterial catheterization (although the catheter flow rate is low).
When loss of a pulse occurs, heparin is continuously infused until the pulse returns or for 12 to 24 hours. There is likely a component of arterial spasm, and heparin may prevent thrombus formation at the site of spasm. If the pulse does not return, treatment with streptokinase or tissue plasminogen activator (tPA) may be instituted unless contraindications are present.46
After Surgery
-
Postoperatively, mechanical ventilation is continued until adequate cardiac output is established (strong peripheral pulses, capillary refill of < 3 seconds with warm distal extremities).47
-
Following Blalock-Taussig shunt (subclavian artery to pulmonary artery)—classic: end-to-side, no or reduced ipsilateral arm pulses; current: side-to-side tubular grafts, preserved arm pulses.
-
Following the Damus-Kaye-Stansel operation: Anecdotal experience suggests that the native aortic valve regurgitation is common in this group of patients, perhaps because of the lack of antegrade flow across the valve combined with the continued retrograde pressure load. This type of regurgitation has physiologic sequelae of a wide pulse pressure and increased volume load on the systemic ventricle and results in a left-to-right shunt and pulmonary overcirculation.48
During Cardiopulmonary Resuscitation (CPR)
Palpation of either the femoral or carotid pulse is a method to know the adequacy of CPR compression.
Pulseless Electrical Activity
Pulseless electrical activity, formerly called electromechanical dissociation, is separated into primary and secondary forms. The common denominator in both is continued electrical rhythmicity of the heart in the absence of effective mechanical function. The secondary form includes the causes that result from an abrupt cessation of cardiac venous return, such as massive pulmonary embolism, acute malfunction of prosthetic valves, exsanguination, and cardiac tamponade from hemopericardium. Electrical mechanisms of cardiac arrest are divided into tachyarrhythmic and bradyarrhythmia-asystolic events.49
Patients who have a bradyarrhythmia or asystole, or pulseless electrical activity, at initial contact have the worst prognosis; only 9% of such patients in the Miami study were admitted to the hospital alive, and none was discharged.
Intermittent Loss of Pulses
-
In cardiac tamponade: During inspiration pulse disappears.
-
Cardiac herniation: One clue to the presence of cardiac herniation is sudden loss of pulse when the patient is repositioned, such as when moved to a stretcher.50
Pulse Rate as Component of Score for Prognostication
In pulmonary embolism: The Pulmonary Embolism Severity Index: PR > 110/min, then score is 20.51
Hypothyroidism
Mild degrees of bradycardia, diastolic hypertension, narrow pulse pressure and relatively quiet precordium, and decreased intensity of the apical impulse are all characteristic.
Acknowledgment
Miss. G. Indrani, PhD student, Department of Cardiology, NIMS—for all the drawn images.
References
- Pulse.Clinical Methods: The History, Physical, and Laboratory Examinations. (3rd ed.). Boston, MA: Butterworths; 1990. In: eds. Available at: https://www.ncbi.nlm.nih.gov/books/NBK278/. Accessed February 28, 2018
- [Google Scholar]
- A history of the heart, cardiac diseases, and the development of cardiovascular medicine.The Heart. New York, NY: McGraw-Hill Medical; 2011. p. :3-6. In: ed.
- [Google Scholar]
- Clinical examination of the arterial pulse. Prog Cardiovasc Dis. 1967;10(03):207-235.
- [Google Scholar]
- Cardiac catheterization and angiography. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :215. eds.
- [Google Scholar]
- Normal physiology of the cardiovascular system.The Heart. New York: McGraw-Hill Medical; 2011. p. :110. In: ed.
- [Google Scholar]
- Pulsatile flow and pressure in human systemic arteries. Studies in man and in a multibranched model of the human systemic arterial tree. Circ Res. 1980;46(03):363-372.
- [Google Scholar]
- History and physical examination. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :60.
- [Google Scholar]
- ST elevation myocardial infarction: pathology, pathophysiology and clinical features. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1102.
- [Google Scholar]
- History and physical examination. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :112-114.
- [Google Scholar]
- Specific arrythmias: diagnosis and treatment. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :796. eds.
- [Google Scholar]
- Physical examination of the arteries and veins.The Heart,. (6th ed.). New York: McGraw-Hill; 1986. p. :138-151. In: ed.
- [Google Scholar]
- Pressure and flow in the arterial and venous systems. In: In: Circulatory Physiology: The Essentials (2nd ed.). Baltimore, MD: Williams and Wilkins; 1984. p. :91-109.
- [Google Scholar]
- Patent ductus arteriosus and aortopulmonary window. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :686-689.
- [Google Scholar]
- Patent ductus arteriosus and aortopulmonary window. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :689.
- [Google Scholar]
- Patent ductus arteriosus and aortopulmonary window. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :692.
- [Google Scholar]
- Patent ductus arteriosus and aortopulmonary window. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :693.
- [Google Scholar]
- Cardiac intensive care. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :453.
- [Google Scholar]
- Coarctation of the aorta. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :991-992.
- [Google Scholar]
- Congenital heart disease. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1455. eds.
- [Google Scholar]
- Hypoplastic left heart Syndrome. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016.
- [Google Scholar]
- Tetralogy of Fallot. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :895.
- [Google Scholar]
- Tricuspid atresia, stenosis, and regurgitation. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :832.
- [Google Scholar]
- Pulmonary atresia and ventricular septal defect. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :881.
- [Google Scholar]
- Truncus arteriosus. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :914.
- [Google Scholar]
- Transposition of the great arteries. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1054.
- [Google Scholar]
- Congenital anomalies of the coronary vessels and the aortic root. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :712.
- [Google Scholar]
- Aortic arch anomalies. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :756.
- [Google Scholar]
- Aortic arch anomalies. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :747.
- [Google Scholar]
- Aortic arch anomalies. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :749.
- [Google Scholar]
- Valvular heart disease. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1481. eds.
- [Google Scholar]
- Rheumatic fever and rheumatic heart disease. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1264.
- [Google Scholar]
- Valvular heart disease. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1473-1474. eds.
- [Google Scholar]
- Aortic stenosis. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :978.
- [Google Scholar]
- Aortic stenosis. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :983.
- [Google Scholar]
- Hypertrophic cardiomyopathy. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1183.
- [Google Scholar]
- Hypertrophic cardiomyopathy. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1588. eds.
- [Google Scholar]
- Hypertrophic cardiomyopathy.The Heart. New York: McGraw-Hill Medical; 2011. p. :841. In: ed.
- [Google Scholar]
- Pericardial diseases. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1291.
- [Google Scholar]
- Pericardial diseases. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1656.
- [Google Scholar]
- Pericardial diseases. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1670.
- [Google Scholar]
- Vascular anomalies. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :720.
- [Google Scholar]
- Vascular anomalies. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :723.
- [Google Scholar]
- Diseases of aorta. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1321-1322.
- [Google Scholar]
- Peripheral arterial disease. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1355.
- [Google Scholar]
- Inflammatory non-infectious cardiovascular diseases. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1288.
- [Google Scholar]
- Cardiac catheterization and angiography. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :236.
- [Google Scholar]
- Cardiac intensive care. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :452.
- [Google Scholar]
- Transposition of the great arteries. In: In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult (9th ed.). Philadelphia, PA: Wolters Kluwer Health; 2016. p. :1081.
- [Google Scholar]
- Cardiac arrest and sudden cardiac death. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :861-864.
- [Google Scholar]
- Traumatic heart diseases. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1675.
- [Google Scholar]
- Pulmonary embolism. In: In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (10th ed.). Philadelphia, PA: Elsevier/Saunders; 2015. p. :1687.
- [Google Scholar]






