Translate this page into:
Amalgamation of Circadian Clock Gene with Incidence of Myocardial Infarction
*Corresponding author: Ghizal Fatima, Department of Biotechnology, Era University, Era’s Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India. ghizalfatima8@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Fatima G, Parvez S, Tuomainen P, Fedacko J, Kazmi DH, Nagib Elkilany GE. Amalgamation of Circadian Clock Gene with Incidence of Myocardial Infarction. Indian J Cardiovasc Dis Women. doi: 10.25259/IJCDW_69_2023
Abstract
Objectives:
The present study included 40 participants to investigate the association of circadian locomotor output cycles kaput (CLOCK) rs4580704 polymorphism with myocardial infarction (MI) cases.
Materials and Methods:
In this study, we enrolled 20 male and 20 female cases with MI. Genomic DNA extraction was done from lymphocytes using conventional techniques, employing the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) from lymphocytes. Genotyping was conducted through TaqMan single-nucleotide polymorphism genotyping assays, employing real-time polymerase chain reaction (PCR) on a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). This streamlined approach ensures accurate and efficient analysis of genetic markers associated with MI across gender groups.
Results:
The study revealed significant associations between body mass index (BMI), hypertension, obesity, current smoking, and type 2 diabetes among both male and female MI patients. However, age, systolic blood pressure (SBP), and diastolic blood pressure (DBP) did not exhibit significant differences between genders. Analysis of CLOCK rs4580704 polymorphism indicated no variance in genotype and allele frequencies between male and female MI patients. When considering both genders, CLOCK rs4580704 polymorphism was significantly associated with BMI, hypertension, obesity, current smoking, and type 2 diabetes (P = 0.02, P = 0.02, P = 0.04, and P = 0.02, respectively). Nevertheless, logistic regression analysis showed no significant differences among MI cases across the various models of CLOCK rs4580704 polymorphism.
Conclusion:
No significant association was found between CLOCK rs4580704 polymorphism and MI in both genders. However, significant links were identified between this polymorphism and various cardiovascular risk factors including BMI, SBP, DBP, hypertension, obesity, current smoking, and type 2 diabetes in MI cases. These findings underscore the potential influence of CLOCK rs4580704 polymorphism on cardiovascular risk profiles among individuals with MI.
Keywords
Amalgamation
Circadian clock
Clock gene
Myocardial infarction
ABSTRACT IMAGE
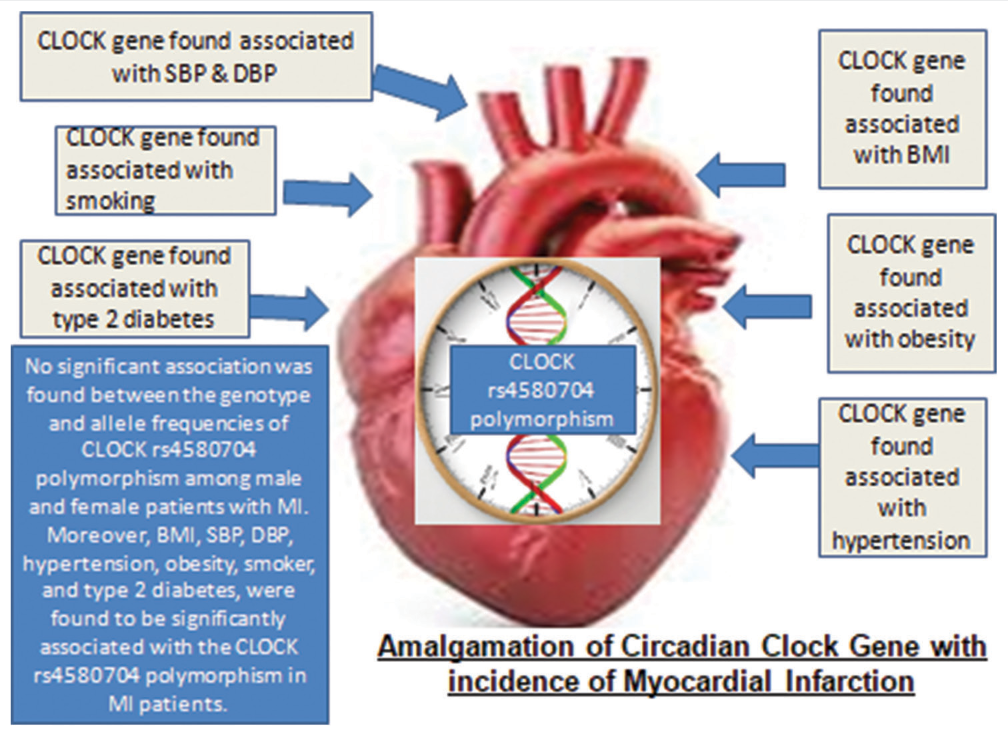
SBP - Systolic Blood Pressure, DBP - Diastolic Blood Pressure, BMI - Body Mass Index, MI - Myocardial Infarction, CLOCK: Circadian locomotor output cycles kaput
INTRODUCTION
Circadian rhythms play a crucial role in regulating metabolic pathways and are closely linked to cardiovascular health. Disruption of these rhythms has been implicated in cardiovascular diseases (CVDs). The circadian rhythm is governed by a complex feedback loop involving core clock genes, with CLOCK being one of them. Studies have suggested a connection between variations in the CLOCK gene and conditions like hypertension, indicating its relevance to cardiovascular risk.[1] Cardiovascular events often exhibit circadian patterns, with a higher incidence observed in the morning.[2] Factors such as myocardial infarction (MI), aortic aneurysm dissection, and stroke tend to occur more frequently in the morning hours.[3-5] Moreover, MI rates are often elevated during winter months, particularly among the elderly population.[6] The circadian rhythm influences various physiological processes, including glucose homeostasis, blood pressure regulation, and vascular function, all of which can impact the onset of MI.[7] The primary circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, with light serving as a key regulator. Disruptions in sleep patterns, whether due to reduced exposure to daylight or excessive nighttime light exposure, can disturb circadian rhythms and contribute to conditions such as obesity, insulin resistance, and hypertension. Sleep disorders are associated with an increased risk of various cardiovascular conditions, including MI.[8-10] Peripheral clocks found in cardiovascular tissues, such as cardiomyocytes and vascular endothelial cells, are synchronized by the central clock in the SCN.[11] These peripheral clocks regulate metabolic gene expression in cardiomyocytes, ensuring coordination with nutrient availability.[12,13] Genetic variations in clock genes have been linked to obesity, sleep disorders, and CVDs such as stroke and MI. Dysregulation of the circadian rhythm can lead to metabolic disorders, dyslipidemia, glucose intolerance, and hypertension, all of which are risk factors for CVD.[14,15] At the molecular level, circadian rhythm genes control gene transcription and protein synthesis through feedback loops involving activators such as CLOCK and aryl hydrocarbon receptor nuclear translocator like (ARNTL), and inhibitors such as period circadian regulator (PER) and cryptochrome (CRY) proteins.[15,16] These proteins regulate the expression of clock-controlled genes, maintaining circadian oscillations that persist for approximately 24 h. While associations between CLOCK gene variations and cardiovascular events have been explored in cross-sectional studies, longitudinal studies are needed to further investigate these relationships.[17-20] In our study, we examined the association between a specific single-nucleotide polymorphism (SNP) (CLOCK-rs4580704) and the incidence of CVD, particularly non-fatal acute MI, in a cohort of 40 individuals, 20 males and 20 females, aged 30–50 years. Our hypothesis was that polymorphisms in circadian clock genes, such as CLOCK, may be linked to MI risk.
Objectives
To investigate the CLOCK gene variation in MI patients, men and women.
To find out the association in between the CLOCK-rs4580704 SNP and incidence of MI.
MATERIALS AND METHODS
The data were collected from a total of 40 individuals, comprising 20 males and 20 females, who had experienced non-fatal acute MI at the Department of CVDs and Intensive Care at Era University. The study adhered to ethical standards and was approved by the Institutional Ethical Review Committee of Era University. All eligible participants provided written informed consent before enrollment. Inclusion criteria for MI patients followed the guidelines established by Thygesen et al.,[21] which define type 1 and 2 MI based on elevated cardiac troponin T levels above the 99th percentile, coupled with symptoms of myocardial ischemia, electrocardiogram changes, pathological Q waves, evidence of myocardial tissue damage, or coronary thrombus presence. Individuals who had undergone percutaneous coronary intervention (PCI) or coronary artery bypass were excluded, as these procedures are not indicative of acute MI. Patients classified as having type 4 and 5 MI were also excluded, along with those who had undergone PCI for type 1 and 2 MI. The control group, consisting of individuals without a documented history of CVD, was selected by cardiologists during routine outpatient visits. Patients with known cardiovascular conditions or a history of MI were excluded from the control group. In addition, relatives of MI patients were excluded from the control group to account for potential genetic predispositions.
Comprehensive medical history data were collected from all participants, including information on age, smoking habits, hypertension, and diabetes mellitus. The study protocol underwent rigorous ethical review and approval from Era University to ensure adherence to ethical guidelines and participant safety. The exclusion criteria were carefully defined to ensure the homogeneity of the study population and to minimize confounding variables that could affect the study outcomes. By excluding individuals who had undergone interventions not indicative of acute MI and those with types 4 and 5 MI, the study focused specifically on type 1 and 2 MI cases, which are characterized by acute myocardial injury due to ischemia. This targeted approach aimed to enhance the reliability and validity of the study findings. Informed consent was obtained from all participants, emphasizing their voluntary participation and the confidentiality of their data. The research team followed strict ethical protocols throughout the study, prioritizing participant welfare and compliance with ethical standards. The collection of systematic medical history data allowed for the comprehensive characterization of the study population, enabling the identification of potential confounders and the adjustment of statistical analyses as necessary. Variables such as age, smoking status, hypertension, and diabetes mellitus were included in the questionnaire to assess their potential influence on the association between the CLOCK-rs4580704 SNP and the incidence of MI. Overall, the study design was meticulously planned and executed, with careful consideration given to ethical principles, participant selection criteria, and data collection methods. By adhering to rigorous ethical standards and employing robust methodologies, the research aimed to provide valuable insights into the relationship between circadian clock gene polymorphisms and CVD risk.
SNP selection and genotyping
In our study, we focused on SNPs within the circadian rhythm gene CLOCK. These SNPs were chosen based on their known genetic linkage and consistency with data from HapMap Phase 3. To identify the most relevant polymorphisms for the CLOCK gene, we utilized the Tagger algorithm, which is integrated into the Haploview software (version 4.2). This algorithm assists in selecting SNPs that capture the genetic variation within a specific gene or genomic region. The genomic DNA required for genotyping was extracted from lymphocytes using standard methods, employing the QIAamp DNA Blood Mini Kit from Qiagen, based in Hilden, Germany.[22] This extraction process ensured the isolation of high-quality DNA suitable for downstream analysis. Genotyping of the selected CLOCK SNPs was carried out using TaqMan SNP genotyping assays, a widely used method known for its accuracy and efficiency. The genotyping assays were performed using the real-time polymerase chain reaction (PCR) technique, utilizing the 7500 Real-Time PCR System from Applied Biosystems, located in Foster City, CA, USA. Real-time PCR offers several advantages for SNP genotyping, including high specificity, sensitivity, and reproducibility. It allows for the simultaneous amplification and detection of specific DNA sequences, enabling efficient genotyping of multiple samples within a short timeframe. The TaqMan SNP genotyping assays utilize allele-specific probes labeled with fluorescent dyes, enabling the discrimination of different SNP alleles during the PCR amplification process. By measuring the fluorescence signals emitted during each PCR cycle, the genotypes of the samples can be accurately determined in real-time. This genotyping approach is particularly suitable for large-scale genetic studies, as it offers high-throughput capabilities and requires minimal hands-on time. Furthermore, the use of standardized genotyping assays ensures consistency and reliability across different laboratories and experimental setups. Overall, the combination of the Tagger algorithm for SNP selection, the QIAamp DNA Blood Mini Kit for DNA extraction, and TaqMan SNP genotyping assays coupled with real-time PCR provided a robust and efficient platform for investigating the association between CLOCK gene polymorphisms and the incidence of CVDs, such as MI, in our study population.
Statistical analysis according to data collected
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 22.0, SPSS Inc., Chicago, IL, USA) for Windows). Quantitative demographic and clinical variables were presented as mean and standard deviation (SD), while categorical variables were presented as frequency and percentages. Chi-square tests (χ2) on contingency tables were applied to analyze allele and genotype frequencies in both groups. By comparing the distribution of genotypes with those expected by the Hardy– Weinberg equilibrium, a further level of quality control of genotyping was accomplished by applying the Chi-square goodness-of-fit test, which was performed using SNPStats[23] and the SHEsis web tools.[24] The relationship between genotypes and cardiovascular risk factors was studied applying the Mann–Whitney U-test. Logistic regression, for the likelihood of a patient having a manifestation of MI, was applied to evaluate the outcome of CLOCK (-rs4580704 SNP) genotypes. Age, hypertension history, diastolic and systolic blood pressure, and body mass index (BMI) were applied as covariates. The Kruskal–Wallis test was applied to establish the association between genotypes and risk factors. When P ≤ 0.05, the associations were marked significant. Appling the Benjamini–Hochberg (false detection rate value) correction method, significant value corrections were made.
RESULTS
The baseline clinical and demographical parameters of the study patients with MI are shown in Table 1. Age, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension, obesity, current smoking, and type 2 diabetes were analyzed. Among the clinical and demographical parameters of MI patients, BMI, hypertension, obesity, current smoking, and type 2 diabetes were found to be significant association between male and female cases. Age, SBP, and DBP were not associated between the male and female MI patients.
| Characteristic | Male (n=20) | Female (n=20) | P-value |
|---|---|---|---|
| Age (years), Mean±SD | 37.2±11.5 | 33.9±8.6 | 0.31 |
| BMI (kg/m2), Mean±SD | 22.8±3.7 | 26.0±5.5 | 0.04* |
| SBP (mmHg), Mean±SD | 127±7.1 | 123.5±6.9 | 0.12 |
| DBP (mmHg), Mean±SD | 82.7±6.1 | 81±6.6 | 0.39 |
| Hypertension (%) | 16(80%) | 8(40%) | 0.02* |
| Obesity cases (%) | 13(65%) | 19(95%) | 0.04* |
| Current smoker (%) | 15(75%) | 1(5%) | 0.0001* |
| Type 2 diabetes (%) | 18(90%) | 11(55%) | 0.03* |
Between-group difference is significant, *P≤0.05. BMI: Body mass index, DBP: Diastolic blood pressure, SBP: Systolic blood pressure, MI: Myocardial infarction, SD: Standard deviation
Genotypic and allelic distributions of the CLOCK rs4580704 polymorphism are shown in Table 2. Genotype frequencies of CLOCK rs4580704 polymorphism showed no difference between male and female patients with MI (P = 0.25). However, CC and GG genotype was significantly higher in females than in males. No difference was observed for allele frequencies between male and female patients with MI (P = 0.65). Although, C allele was found to be higher in male MI patients as compared to female patients [Table 3]. This table provides mean values (±SD) for continuous variables such as age, BMI, SBP, and DBP across different genotypes (CC, CG, and GG) of the CLOCK rs4580704 polymorphism. It also presents the frequency and percentage of categorical variables including hypertension, obesity cases, current smoking status, and type 2 diabetes across the three genotypes. Here, P-values indicate the statistical significance of differences observed between genotypes for each characteristic. For continuous variables (age, BMI, SBP, and DBP), P-values less than the chosen significance level (typically 0.05) suggest statistically significant differences between groups. Similarly, for categorical variables (hypertension, obesity cases, current smoker, and type 2 diabetes), P < 0.05 indicates significant differences in the frequency of these characteristics between genotypes. Table 3 provides valuable insights of CLOCK rs4580704 polymorphism association with various clinical and demographic parameters, shedding light on potential risk factors and underlying mechanisms involved in MI susceptibility.
| Genotypes | Male n=20 (%) | Female n=20 (%) | P-value | X2 |
|---|---|---|---|---|
| CC | 9 (45) | 10 (50) | 0.25 | 2.74 |
| CG | 6 (30) | 2 (10) | ||
| GG | 5 (25) | 8 (40) | ||
| Alleles | ||||
| C | 24 (60) | 22 (55%) | 0.65 | 0.20 |
| G | 16 (40) | 18 (45%) |
MI: Myocardial infarction, CLOCK: Circadian locomotor output cycles kaput
| Characteristic | CC=19 | CG=8 | GG=13 | P-value |
|---|---|---|---|---|
| Age (years), Mean±SD | 37.8±11.4 | 31.5±6.8 | 34.6±9.5 | 0.32 |
| BMI (kg/m2), Mean±SD | 22.5±1.7 | 23.8±2.2 | 27.6±7.4 | 0.01* |
| SBP (mmHg), Mean±SD | 126.5±7.0 | 128.1±7.5 | 121.5±5.9 | 0.06 |
| DBP (mmHg), Mean±SD | 82.6±6.3 | 81.8±7.0 | 80.7±6.4 | 0.07 |
| Hypertension (%) | 13 (54.2) | 7 (29.2) | 4 (16.7) | 0.02* |
| Obesity cases (%) | 18 (56.3) | 4 (12.5) | 10 (31.3) | 0.02* |
| Current smoker (%) | 12 (66.7) | 1 (5.6) | 5 (27.8) | 0.04* |
| Type 2 diabetes (%) | 17 (58.6) | 3 (10.3) | 9 (31) | 0.02* |
Between-group difference is significant *P≤0.05. BMI: Body mass index, DBP: Diastolic blood pressure, SBP: Systolic blood pressure, MI: Myocardial infarction, SD: Standard deviation, CLOCK: Circadian locomotor output cycles kaput
[Table 4] Each section of the table represents different models of genetic inheritance (co-dominant, dominant, and recessive) for the CLOCK rs4580704 genotype. The table provides the genotype frequencies for male and female MI patients, along with the corresponding risk ratios, odds ratios with 95% confidence intervals, and P-values calculated from logistic regression analysis for each genotype model. The results indicate the association between CLOCK rs4580704 genotype and MI risk, considering different genetic inheritance models.
| Genotype | Male n=20 (%) | Female n=20 (%) | Risk ratio | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Co-dominant model | |||||
| CC | 9 (45) | 10 (50) | 1 | 1 | |
| CG | 6 (30) | 2 (10) | 0.63 (0.33–1.17) | 0.30 (0.04–1.88) | 0.37 |
| GG | 5 (25) | 8 (40) | 1.23 (0.52–2.84) | 1.44 (0.34–6.05) | 0.89 |
| Dominant model | |||||
| CC | 9 (45) | 10 (50) | 1 | 1 | 0.75 |
| CG+GG | 11 (55) | 10 (50) | 0.90 (0.48–1.69) | 0.81 (0.23–2.83) | |
| Recessive model | |||||
| CC+CG | 15 (75) | 12 (60) | 1 | 1 | 0.49 |
| GG | 5 (25) | 8 (40) | 1.44 (0.67–3.10) | 2.0 (0.51–7.72) |
Between-group difference is significant P≤0.05. OR: odds ratios, CI: Confidence intervals , CLOCK: Circadian locomotor output cycles kaput
DISCUSSION
MI is a complex condition characterized by a progressive decline in cardiac function, leading to impaired pump function and insufficient blood flow to meet the body’s demands. Identifying risk factors and potential genetic determinants associated with MI is crucial for the early intervention and preventive strategies. In this study, we investigated the association of the CLOCK rs4580704 polymorphism with MI cases, aiming to shed light on the genetic predisposition to MI and its early detection. In this study, we had included 40 participants to investigate the association of CLOCK rs4580704 polymorphism with MI cases. To the best of our knowledge, this work will be the first investigation to explore and clarify the association of CLOCK rs4580704 polymorphism and the incidence of MI in north Indian patients. The results are in agreement with previous knowledge obtained from animal and human cross-sectional studies. In our study, we found that the CLOCK rs4580704 polymorphism is significantly associated with obesity and type 2 diabetes. In agreement to our results, Garaulet et al., reported significant associations with weight and BMI for SNPs rs4580704.[25] The findings from our study revealed several noteworthy observations regarding the relationship between the CLOCK rs4580704 polymorphism and MI risk. While previous studies have suggested a potential link between genetic variations in the CLOCK gene and CVDs, including MI, our results did not show a significant association between the genotype and allele frequencies of the CLOCK rs4580704 polymorphism among male and female patients with MI. This finding contradicts some earlier studies suggesting a possible role of the CLOCK gene in cardiovascular pathophysiology. However, it is essential to interpret these results cautiously, considering potential confounding factors and the need for further investigation in larger cohorts.
Moreover, our results are supported by observations on murine models, given that mice with mutations resulting in under-expression of the clock gene presented obesity and hyperglycemia.[26,27] Moreover, CLOCK rs4580704 polymorphism was associated with metabolic syndrome,[25] diabetes,[28] obesity,[29] and CVDs.[29] Similarly, our study and previous epidemiological studies[25,28,30,31] show that the CLOCK rs4580704 polymorphism was also significantly associated with BMI. Despite the lack of a significant association between the CLOCK rs4580704 polymorphism and MI risk, our study identified several clinical parameters that were significantly associated with both the genotype and allele frequencies of the CLOCK rs4580704 polymorphism. These parameters included BMI, hypertension, obesity, current smoking status, and type 2 diabetes. The observed associations suggest a potential interplay between genetic predisposition and environmental factors in the pathogenesis of chronic heart failure (CHF). Specifically, individuals carrying certain CLOCK gene variants may be more susceptible to the detrimental effects of these risk factors, leading to an increased risk of MI.
The association of the CLOCK rs4580704 polymorphism with clinical parameters such as BMI, hypertension, obesity, smoking, and type 2 diabetes underscores the multifactorial nature of CHF. These findings highlight the importance of considering both genetic and environmental factors in risk assessment and disease management strategies. Furthermore, the identification of specific risk factors associated with genetic variations in the CLOCK gene may aid in risk stratification and personalized approaches to CHF prevention and treatment. One of the notable findings of our study was the lack of a significant association between age, SBP, DBP, and the CLOCK rs4580704 polymorphism in male and female MI patients. While age and blood pressure are well-established risk factors for CVDs, including CHF, our results suggest that the CLOCK gene variant examined in this study may not directly influence these parameters. However, it is essential to note that our study sample size may have limited the statistical power to detect small effect sizes. In addition, our study investigated the association between different genetic inheritance models (co-dominant, dominant, and recessive) of the CLOCK rs4580704 polymorphism and MI risk. While no significant associations were observed in the codominant and dominant models, the recessive model showed a trend toward significance, particularly for the GG genotype. This finding suggests a potential recessive mode of inheritance for the CLOCK rs4580704 polymorphism in MI risk, although larger studies are needed to confirm this observation.
CONCLUSION
Our study provides insights into the genetic and clinical factors associated with MI risk, particularly in the context of the CLOCK rs4580704 polymorphism. While we did not find a significant association between the genotype and allele frequencies of this polymorphism and MI risk, our findings highlight the importance of considering genetic variations and environmental factors in MI pathogenesis. Further, research in larger, well-characterized cohorts is needed to validate our findings and elucidate the underlying mechanisms linking genetic predisposition to MI development. Ultimately, a better understanding of the genetic determinants of MI may lead to improved risk prediction, prevention, and personalized treatment strategies for this debilitating condition.
Ethical approval
The research/study approved by the Institutional Review Board at ERA UNIVERSITY, number 210, dated February 20, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Audio summary available at
Financial support and sponsorship
WINCARS.
References
- Molecular Architecture of the Mammalian Circadian Clock. Trends Cell Biol. 2014;24:90-9.
- [CrossRef] [PubMed] [Google Scholar]
- Circadian and Seasonal Variations of Physiological and Biochemical Determinants of Acute Myocardial Infarction. Biol Rhythm Res. 2007;38:169-79.
- [CrossRef] [Google Scholar]
- Circadian Variation in the Frequency of Onset of Acute Myocardial Infarction. N Engl J Med. 1985;313:1315-22.
- [CrossRef] [PubMed] [Google Scholar]
- Ambulatory Blood Pressure Monitoring and Sleep Quality in Hypertensive Men and Women. Indian J Cardiovasc Dis Women. 2023;8:187-92.
- [CrossRef] [Google Scholar]
- Circadian Variation in the Timing of Stroke Onset: A Meta-Analysis. Stroke. 1998;29:992-6.
- [CrossRef] [PubMed] [Google Scholar]
- Seasonal Variation in Onset of Myocardial Infarction-A 7-Year Single-center Study in Italy. Chronobiol Int. 2005;22:1121-35.
- [CrossRef] [PubMed] [Google Scholar]
- Chronobiology, Genetics and Metabolic Syndrome. Curr Opin Lipidol. 2009;20:127-34.
- [CrossRef] [PubMed] [Google Scholar]
- Circadian Influences on Myocardial Infarction. Front Physiol. 2014;5:422.
- [CrossRef] [Google Scholar]
- Association of Circadian Genes with Diurnal Blood Pressure Changes and Non-dipper Essential Hypertension: A Genetic Association with Young-onset Hypertension. Hypertens Res. 2015;38:155-62.
- [CrossRef] [PubMed] [Google Scholar]
- Circadian Gene Variants and Susceptibility to Type 2 Diabetes: A Pilot Study. PLoS One. 2012;7:e32670.
- [CrossRef] [PubMed] [Google Scholar]
- NPAS2 and PER2 are Linked to Risk Factors of the Metabolic Syndrome. J Circadian Rhythm. 2009;7:5.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of the Circadian Rhythm on Microvascular Function in Patients with ST-Elevation Myocardial Infarction. Int J Cardiol. 2013;168:4948-9.
- [CrossRef] [PubMed] [Google Scholar]
- Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism. 2018;84:11-27.
- [CrossRef] [PubMed] [Google Scholar]
- Daylight Saving Time, Circadian Rhythms, and Cardiovascular Health. Intern Emerg Med. 2018;13:641-6.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of the Cardiomyocyte Circadian Clock on Cardiac Physiology and Pathophysiology. J Biol Rhythm. 2015;30:183-205.
- [CrossRef] [PubMed] [Google Scholar]
- The Cardiomyocyte Circadian Clock: Emerging Roles in Health and Disease. Circ Res. 2010;106:647-58.
- [CrossRef] [PubMed] [Google Scholar]
- Chronobiological Aspects of Obesity and Metabolic Syndrome. Endocrinol Nutr. 2012;59:50-61.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Circadian Rhythm with Myocardial Infarction. Acta Clin Croat. 2018;57:480-5.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic Causes of Myocardial Infarction: New Insights from Genome-wide Association Studies. Dtsch Arztebl Int. 2010;107:694-9.
- [CrossRef] [PubMed] [Google Scholar]
- Circadian Clock and the Onset of Cardiovascular Events. Hypertens Res. 2016;39:383-90.
- [CrossRef] [PubMed] [Google Scholar]
- Third Universal Definition of Myocardial Infarction. Nat Rev Cardiol. 2012;9:620-33.
- [CrossRef] [PubMed] [Google Scholar]
- Efficiency and Power in Genetic Association Studies. Nat Genet. 2005;37:1217-23.
- [CrossRef] [PubMed] [Google Scholar]
- SNPStats: A Web Tool for the Analysis of Association Studies. Bioinformatics. 2006;22:1928-9.
- [CrossRef] [PubMed] [Google Scholar]
- SHEsis, a Powerful Software Platform for Analyses of Linkage Disequilibrium, Haplotype Construction, and Genetic Association at Polymorphism Loci. Cell Res. 2005;15:97-8.
- [CrossRef] [PubMed] [Google Scholar]
- CLOCK Genetic Variation and Metabolic Syndrome Risk: Modulation by Monounsaturated Fatty Acids. Am J Clin Nutr. 2009;90:1466-75.
- [CrossRef] [PubMed] [Google Scholar]
- BMAL1 and CLOCK, Two Essential Components of the Circadian Clock, are Involved in Glucose Homeostasis. PLoS Biol. 2004;2:e377.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science. 2005;308:1043-5.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic Variants of Clock Transcription Factor are Associated with Individual Susceptibility to Obesity. Am J Clin Nutr. 2008;87:1606-15.
- [CrossRef] [PubMed] [Google Scholar]
- CLOCK Gene Variation is Associated with Incidence of Type-2 Diabetes and Cardiovascular Diseases in Type-2 Diabetic Subjects: Dietary Modulation in the PREDIMED Randomized Trial. Cardiovasc Diabetol. 2016;15:4.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Polymorphisms in the Clock Gene, Obesity and the Metabolic Syndrome in Man. Int J Obes (Lond). 2008;32:658-62.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Genetic Variants of the Clock Gene and Obesity and Sleep Duration. J Physiol Biochem. 2015;71:855-60.
- [CrossRef] [PubMed] [Google Scholar]







