Translate this page into:
Acute MI Versus Takotsubo Cardiomyopathy
S. V. V. Mani Krishna, DM Department of Cardiology NIMS, Punjagutta, Hyderabad – 500082 India manikrishnas009@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
A 37-year-old female, who was diagnosed with rheumatoid arthritis (RA) 20 years earlier and on regular treatment, with a recent history of pulmonary thromboembolism on inj. clexane, presented with anginal type of chest pain of 4 hours duration ECG, showing ST elevation, and was finally diagnosed to have Takotsubo cardiomyopathy.
Keywords
Takotsubo cardiomyopathy
Discussant: Dr. S.V.V. Mani Krishna
The likely differential diagnoses are as follows:
Presenter: A 37-year-old female, who was a known case of RA since 20 years was on regular treatment with disease-modifying antirheumatic drugs (DMARDs) methotrexate and hydroxychloroquine.
She underwent total knee replacement in 2010 for bilateral knees and total hip replacement in 2012. She also had a history of irregular menstrual cycles, with excessive bleeding from the past one year. While evaluation, she was found to have fibroid uterus, which was planned for surgery. Just before surgery, she developed sudden onset of breathlessness associated with chest pain. She was admitted and evaluated at our center and found to have bilateral lower limb deep vein thrombosis (DVT) with submassive pulmonary thromboembolism. As she has to undergo hysterectomy surgery, so she underwent inferior vena cava (IVC) filter implantation through right internal jugular vein (IJV) access and was discharged with inj. clexane 40 mg subcutaneously (SC) twice daily at home.
After 4 days of discharge from hospital, she was again admitted to our hospital with a sudden onset of chest pain of 4-hour duration, retrosternal in location and deep constricting type, radiating to left upper limb, associated with breathlessness and sweating not related to posture and respiration and food intake lasting for 5 minutes Patient did not give any history of orthopnea or paroxysmal nocturnal dyspnea (PND) episodes.
Neither was there any history of palpitations and giddiness episodes nor of syncope or fall.
There was no history of pedal edema, fever, rash, cough, easy fatiguability, weight loss, abdominal distension or pain, generalized anasarca, constipation, diarrhea, melena, tingling numbness of limbs, reduced urine output, burning micturition, or bleeding manifestations.
There was no history of similar episodes in the past. Patient is housewife by profession. Patient consumes a mixed diet. There was no history of alcohol intake, smoking, or tobacco chewing. There was no history of use of any other medication other than drugs prescribed for rheumatoid arthritis (RA), and inj. clexane prescribed for pulmonary thromboembolism. No family history of any cardiac disease or other medical illness. First childbirth involved intrauterine death at term pregnancy. Second childbirth was uneventful, and child is healthy.
Discussant: Summary of the present illness is a 37-year-old female, with a diagnosed case of RA since 20 years and on regular treatment, along with a recent history of pulmonary thromboembolism on inj. clexane, who presented with anginal type of chest pain of 4 hours duration.
Acute Myocardial Infarction
Chest pain here is characteristic of angina. Myocardial ischemia usually occurs in the setting of coronary atherosclerosis, but it may also reflect dynamic components of coronary vascular resistance. Coronary spasm can occur in normal coronary arteries, in patients with coronary artery disease (CAD), near atherosclerotic plaque, and in smaller coronary arteries.1 Other, less common causes of impaired coronary blood flow include syndromes that compromise the orifices or lumina of the coronary arteries, such as coronary arteritis, proximal aortitis, spontaneous coronary artery dissection, proximal aortic dissection, coronary emboli from infectious or noninfectious endocarditis or thrombus in the left atrium or left ventricle, myocardial bridge, or a congenital abnormality of the coronary arteries.
Acute Pulmonary Thromboembolism
Massive pulmonary emboli tend to cause severe and persistent substernal pain, which is attributed to distention of the pulmonary artery.2 Smaller emboli that lead to pulmonary infarction can cause lateral pleuritic chest pain. Hemodynamically significant pulmonary emboli may cause hypotension, Syncope and right-sided heart failure. In our patient, there is no syncope and right-sided heart failure.
Acute Myopericarditis
Pericarditis patients typically experience pleuritic pain with breathing, coughing, and changes in position. Swallowing may induce the pain because of the proximity of the esophagus to the posterior portion of the heart. Because the central diaphragm receives its sensory supply from the phrenic nerve and the phrenic nerve arises from the third to fifth cervical segments of the spinal cord, pain from pericardium is frequently felt in the shoulders and neck. Our patient does not have chest pain characteristic of pericarditis. However, pericarditis occasionally causes a steady, crushing substernal pain resembling that of acute myocardial infarction (AMI).3
Acute Aortic Dissection
Acute aortic dissection usually causes a sudden onset of excruciating ripping pain. Ascending aortic dissection tends to manifest as pain in the midline of the anterior aspect of the chest, and posterior descending aortic dissection tends to cause pain in the back of the chest.4 Our patient does not have any backpain and ripping quality.
Severe PAH Secondary to Rheumatoid Arthritis
As patient is a known case of RA since 20 years, chances of her having pulmonary arterial hypertension (PAH) is high. PAH can result in chest pain, similar to that of angina pectoris, presumably because of right heart hypertrophy and ischemia.5
Takotsubo Cardiomyopathy
Patient had emotional triggers because of multiple surgeries for RA, so chances of developing Takotsubo cardiomyopathy is high. Chest pain is the predominant symptom (around 76%) in Takotsubo cardiomyopathy.6
Spontaneous Pneumothorax
Sudden onset of unilateral pleuritic pain, with dyspnea is known to occur in spontaneous pneumothorax. However, in our patient, chest pain is retrosternal and not unilateral.
Esophageal Spasm
Esophageal spasm can produce a squeezing chest discomfort, similar to that of angina. Patient is also having associated breathlessness, so likely possibility of esophageal spasm is very less.
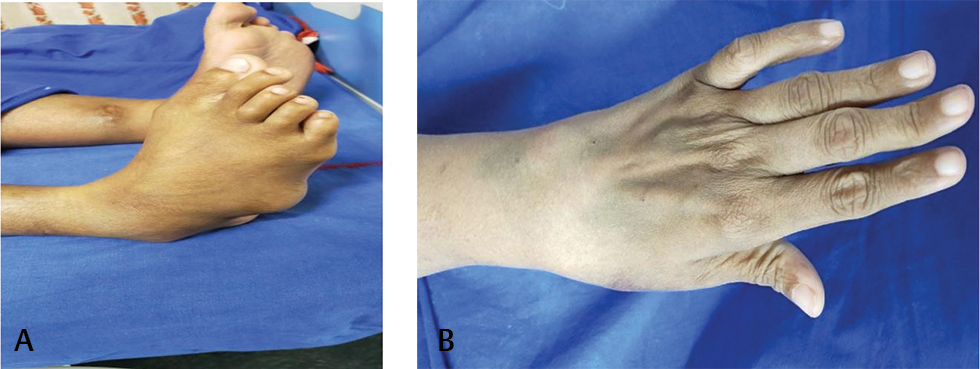
Presenter: On examination, patient was alert and oriented. Her body mass index (BMI) was 32.6 kg/m2. Temperature was 98.4 °F. Pulse rate was 100 beats/min, which was regular; blood pressure was 110/70 mm Hg in right arm supine position. Respiratory rate was 22 cycles per minute and oxygen saturation was 99% while breathing ambient air. There was no pallor, icterus, cyanosis, clubbing, edema feet, or lymphadenopathy. Deformities of small joints of hands and feet were present (Figs. 1 A, B). On cardiovascular system examination, jugular venous pressure (JVP) had normal mean column height and normal waveforms. Apex beat was localized in left 5th intercostal space along midclavicular line, left ventricular (LV) type and diffuse impulse. First heart sound was soft in intensity. LV S3 and S4 are audible. Second heart sound was normal in intensity and split. No adventitious sounds were appreciated. The lungs were clear. There was no organomegaly as per abdomen examination.
-
Fig. 1 (A, B) Showing deformities of small joints of feet and hands.
Fig. 1 (A, B) Showing deformities of small joints of feet and hands.
Discussant: To summarize the examination findings, patient had a pulse rate of 100/min, regular with a normal JVP and soft intensity of first heart sound, and LV S3 and S4 pointing toward AMI as the cause of acute chest pain.
Comprising history and examination findings, the probable diagnosis is as follows:
Acute Myocardial Infarction
Patients with RA are prone to have early atherosclerosis. Systemic inflammatory diseases cause premature atherogenesis. Evidence supporting an association between inflammatory diseases and accelerated atherogenesis is best developed for RA and systemic lupus erythematosus (SLE).7 The risk of myocardial infarction in patients with RA is considered similar to that in those with diabetes mellitus, and women with RA are twice as likely as age-matched controls in the general population to suffer myocardial infarction.8 Coronary arteritis can also occur in RA.
Acute Pericarditis
In more than 90% of cases, the main symptom of acute pericarditis is chest pain, often quite severe. It is usually retrosternal. The trapezius ridge is a classic radiation. Pericardial pain is pleuritic and worsened by lying down. Associated symptoms include dyspnea, cough, and occasionally hiccups.
Clinically, significant pericarditis affects only 1 to 2% of patients with RA, more commonly male, seropositive patients.9 Constrictive pericarditis can develop over a period of months. Hemodynamically significant pericarditis, although reported, is extremely rare in patients being treated with antirheumatic therapy.
Viral Myocarditis: Patient presented with sudden onset shortness of chest pain along with breathlessness. Hence, the possibility of viral myocarditis should be strongly considered. However, no inciting event was reported by the patient.
Takotsubo Cardiomyopathy
Chest pain was the predominant symptom in 76%, dyspnea in 47%, and syncope in 7.7%. A preceding physical trigger occurred in 36% and an emotional trigger in 28%.10
Investigations:
-
Chest X-ray (Fig. 2)
-
ECG

-
Fig. 2 Chest X-ray PA view.
Fig. 2 Chest X-ray PA view.

-
Fig. 3 ECG on hospital admission.
Fig. 3 ECG on hospital admission.
-
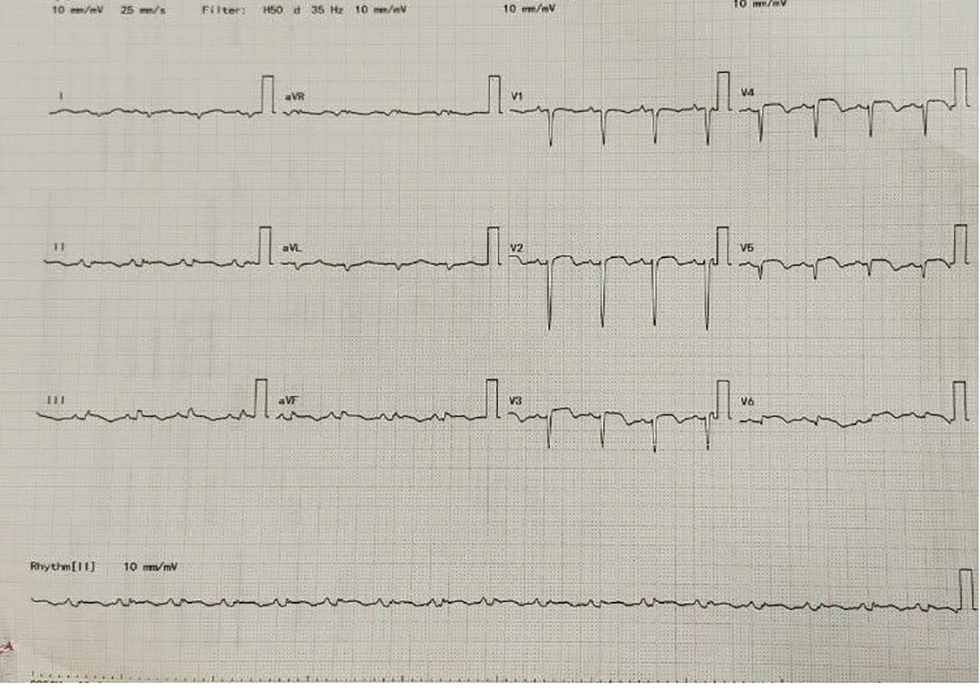
Fig. 4 Follow-up ECG on day 3 of hospital stay after thrombolysis with tenecteplase.
Fig. 4 Follow-up ECG on day 3 of hospital stay after thrombolysis with tenecteplase.

-
Fig. 5 Echo image showing mild left ventricle (LV) dysfunction at the time of admission.
Fig. 5 Echo image showing mild left ventricle (LV) dysfunction at the time of admission.
-
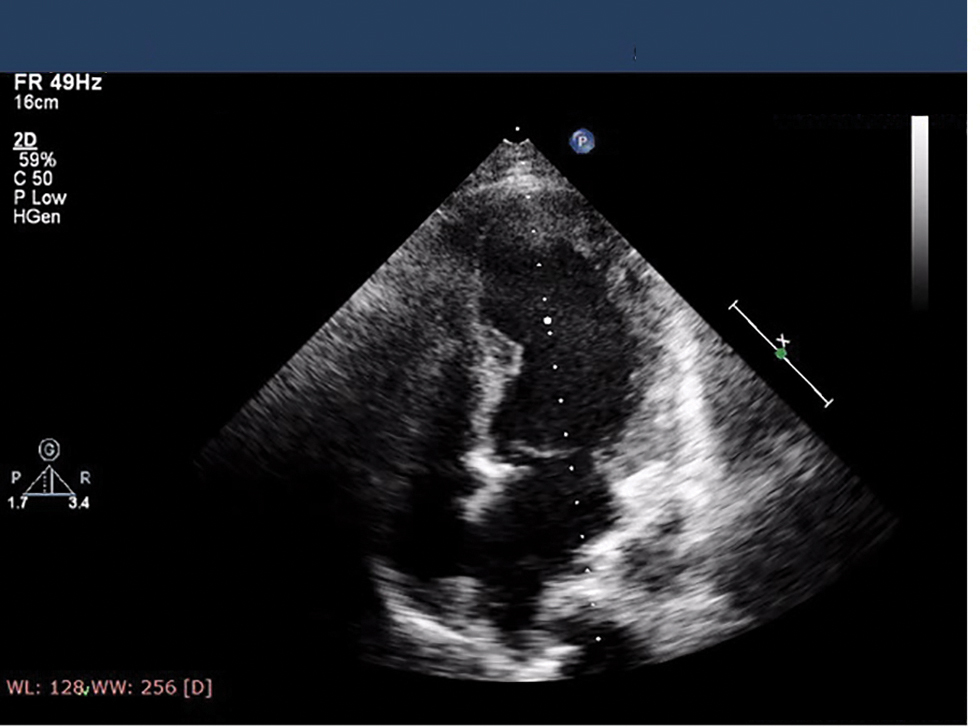
Fig. 6 Showing severe left ventricle (LV) dysfunction on day 2 of admission with apical ballooning.
Fig. 6 Showing severe left ventricle (LV) dysfunction on day 2 of admission with apical ballooning.
|
Biochemical parameter |
Patient range |
|---|---|
|
Abbreviations: ANA, antinuclear antibody; APTT, activated partial thromboplastin time; CPK, creatine phosphokinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HDL, high-density lipoprotein; INR, international normalized ratio; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; PT, prothrombin time; TGC, triglycerides; VLDL, very low density lipoprotein.
|
|
|
Hemoglobin (gm/dl) |
14 |
|
White blood cells (per ul) |
8600 |
|
Platelet count (per ul) |
260000 |
|
Sodium/potassium (mmol/L) |
137/5.1 |
|
Creatinine/urea (mg/dl) |
0.86/50 |
|
Total bilirubin/direct (mg/dl) |
0.6/0.2 |
|
SGOT/SGPT (U/L) |
36/28 |
|
Total protein/albumin (gm/dl) |
8/4.2 |
|
PT/INR/APTT (sec) |
11/1.02/24 |
|
Total cholesterol/TGC (mg/dl) |
152/ 78 |
|
HDL/LDL/VLDL (mg/dl) |
42/84/16 |
|
Hs-CRP (mg/L) |
2 |
|
ESR (mm 1 hour) |
28 |
|
NT pro BNP (pg/ml) |
12116 |
|
ANA profile |
Negative |
|
CPK/LDH (U/L) |
400/243 |
|
Peak troponin T levels |
26 ng/L |
|
Antiphospholipid antibodies |
Negative |
|
Plasma homocysteine |
18 mcmol/L |
Radiologist: Chest X-ray showing cardiomegaly with LV apex (Fig. 1). Aorta is normal, and pulmonary bay is preserved. Grade 1 pulmonary venous hypertension.
Electrophysiologist Opinion
ECG on admission was suggestive of anterior wall AMI with ST elevation in all the precordial leads 1 and augmented Vector Left (aVL) (Fig. 3).
As patient is presented within 4 hours of chest pain, she underwent thrombolysis with tenecteplase. Following thrombolysis, there is resolution of ST elevations (Fig. 4).
Discussant
So, after chest X-ray and ECG, there are three possible differential diagnosis.
-
Acute Myocardial Infarction
Evolution of ECG changes and development of Q waves in our patient points toward myocardial infarction as the possible etiology of chest pain. But there is absence of reciprocal ST segment depressions. There is 2 to 3 mm ST elevation in precordial leads along with Q waves.
-
Acute Pericarditis
Pericarditis will have ST-segment elevation in all leads except augmented Vector Right (aVR) and often V1. Usually, the ST segment is coved upward and resembles transmural ischemia. Both can be differentiated by more extensive lead involvement and lack of evolution to pathologic Q waves in pericarditis, and more prominent reciprocal ST depression in ischemia.11 PR segment depression is also common and considered the earliest ECG sign of acute pericarditis.11 In our case, ECG evolution to Q waves is seen and there is no PR segment depression. So, the possibility of acute pericarditis is less likely.
-
Takotsubo Cardiomyopathy
ST elevation shown on the ECG in almost half of the patients with Takotsubo cardiomyopathy. New and reversible ECG abnormalities (ST-segment elevation, ST depression, LBBB, b T-wave inversion, and/or QTc prolongation) are seen during the acute phase (first 3 months).12
Presenter: 2D echo was done and suggestive of regional wall motion abnormality (RWMA) in anterior wall and apex, mild LV dysfunction ejection fraction (EF) 42%, grade I diastolic dysfunction, no mitral regurgitation (MR)/aortic regurgitation (AR)/tricuspid regurgitation (TR)/PAH, and no pulmonary embolism (PE)/vegetation/clot (Fig. 5).
Presenter: Following thrombolysis with tenecteplase, patient developed profuse vaginal bleeding not controlled by medical therapy. She also developed pulmonary edema requiring continuous positive airway pressure (CPAP) support. 2D echo at that time has shown severe LV dysfunction with apical ballooning (Fig. 6). Gynecology consultation taken for profuse vaginal bleeding were advised uterine artery embolization. Patient underwent successful uterine artery embolization with control of vaginal bleeding.
The possible differential diagnoses based on 2D echo findings are as follows:
Takotsubo Cardiomyopathy
In our case, there is circumferential dysfunction of ventricular segments, and abnormality is not confined to single vascular territory. These features are favoring Takotsubo cardiomyopathy. The RWMAs usually extend beyond a single epicardial vascular distribution and cause circumferential dysfunction of the ventricular segment in case of Takotsubo cardiomyopathy.13 LV contractile abnormalities in Takotsubo cardiomyopathy are prominent, and although they involve the LV apex (resulting in the synonym of “apical ballooning syndrome”) in more than 80% of patients, RWMAs may be limited to the midventricular wall or other LV walls in a minority of patients.13
Acute Myocardial Infarction
As 2D echo is showing wall motion abnormalities in apical areas, even though not confined to single vascular territory, still the possibility of AMI cannot be denied in our case.
Presenter: Laboratory investigations
Except for elevation of cardiac enzymes and NT pro BNP, the rest of the reports were normal.
Takotsubo Cardiomyopathy
In our case, there is disparity between peak troponin levels and amount of dysfunctional myocardium. Also, there exists an inverse relation between NT pro BNP levels and peak troponin T levels, with NT pro BNP levels being significantly elevated compared with troponin T. These features favor Takotsubo cardiomyopathy rather than anterior wall AMI. A positive but relatively small elevation in cardiac troponin can be measured with a conventional assay in Takotsubo cardiomyopathy.14
Acute Myocardial Infarction
The rise in BNP and NT pro BNP after ST-elevation myocardial infarction (STEMI) correlates with infarct size and RWMAs. But, in our patient, there is mild elevation of cardiac troponin T and its levels normalized within 1 week. Because of continuous release from a degenerating contractile apparatus in necrotic myocytes, elevations in cTnI may persist for 7 to 10 days after MI; elevations in cTnT may persist for up to 10 to 14 days.15 These laboratory findings are against the diagnosis of MI.
As levels of inflammatory markers like erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are less in this patient, so the possibility of coronary arteritis as the cause of acute MI is less likely. Antiphospholipid antibodies are also negative in our patient, so the possibility of antiphospholipid syndrome causing coronary thrombosis is ruled out.
Coronary Angiogram
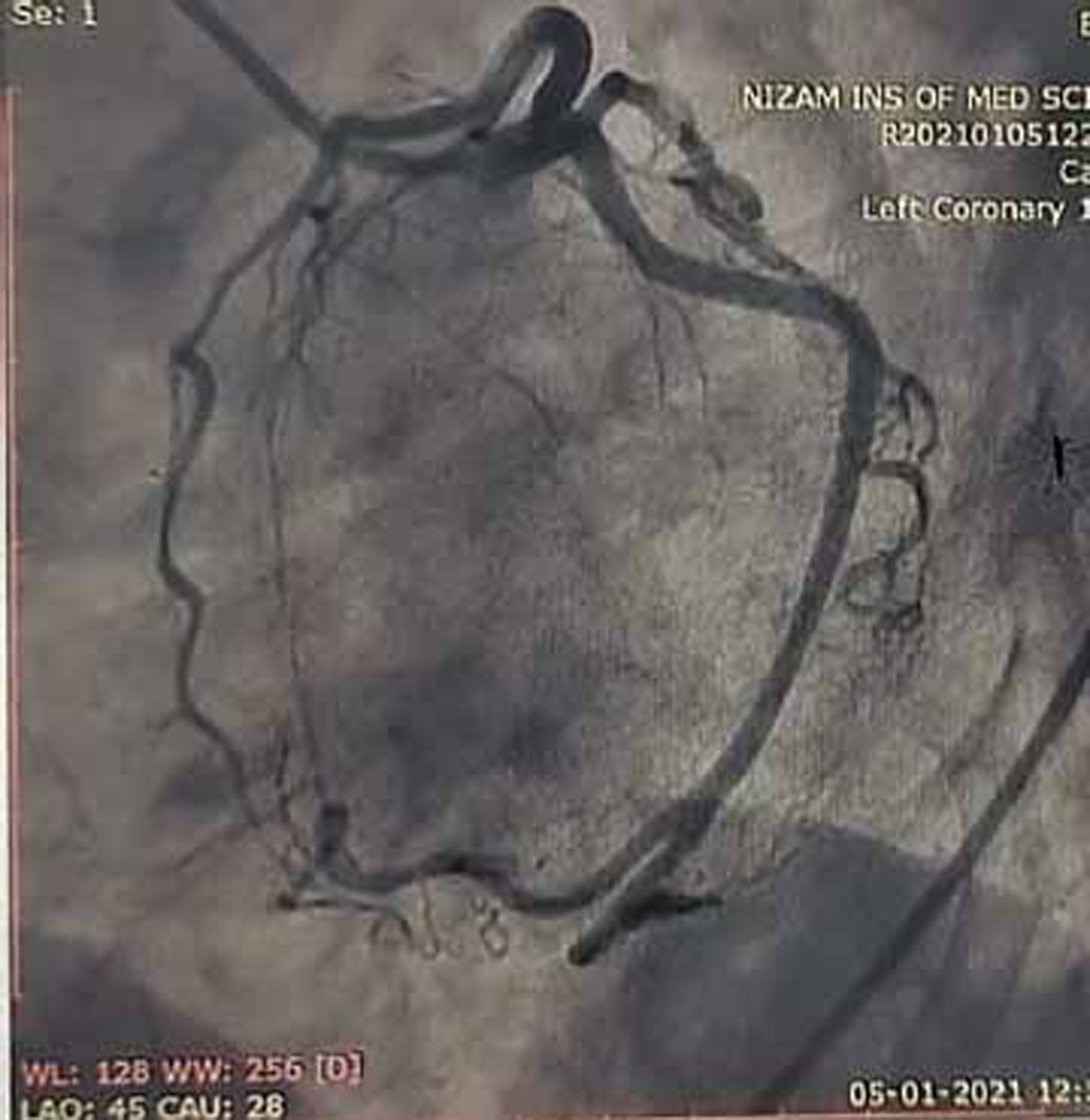
Coronary angiogram (CAG) was done and showed normal epicardial coronaries (Figs. 7 and 8).
-
Fig. 7 Coronary angiogram (CAG) in left anterior oblique (LAO) caudal view showing normal proximal left anterior descending (LAD) artery and proximal left circumflex artery (LCX) which are normal.
Fig. 7 Coronary angiogram (CAG) in left anterior oblique (LAO) caudal view showing normal proximal left anterior descending (LAD) artery and proximal left circumflex artery (LCX) which are normal.
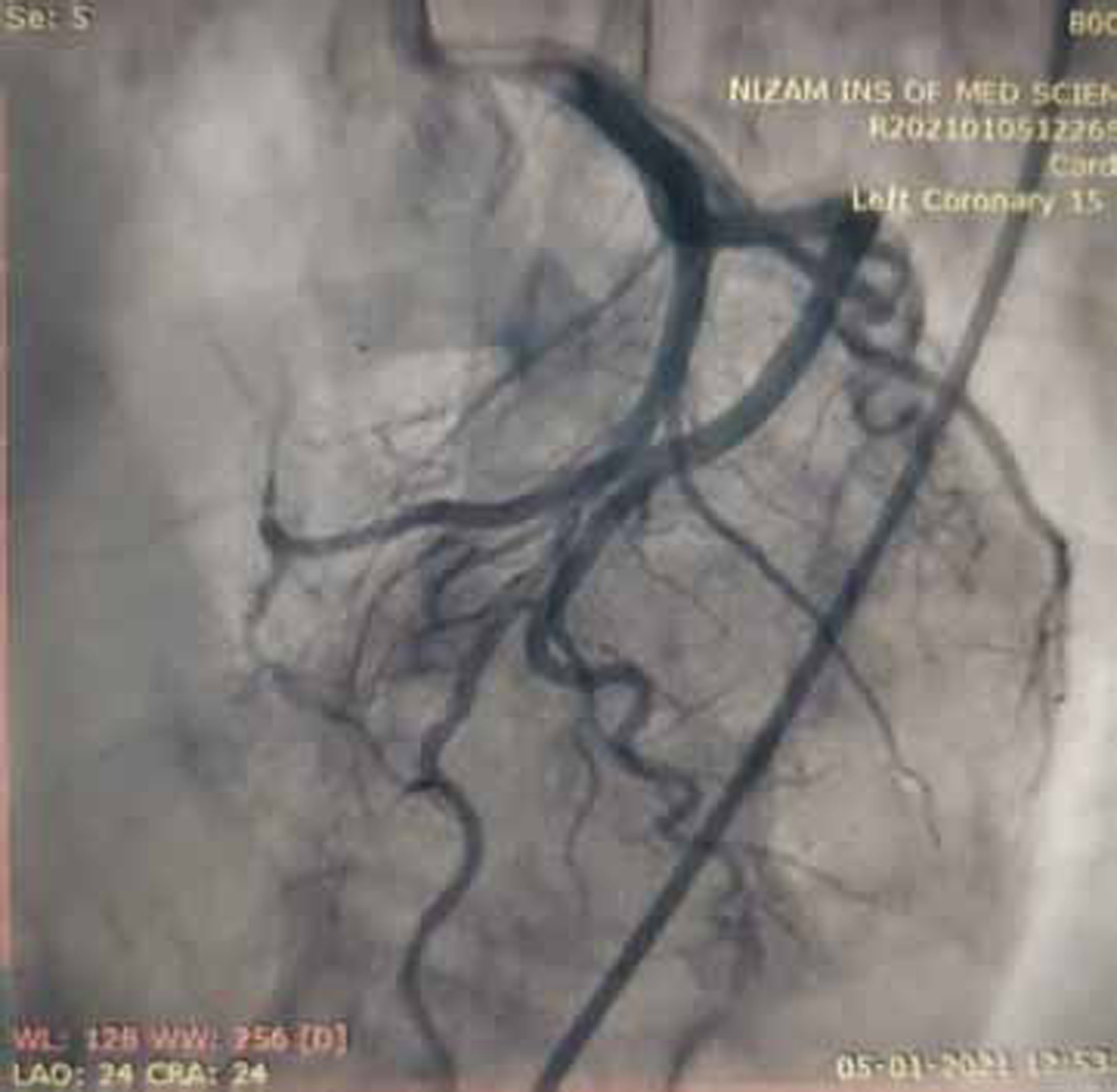
-
Fig. 8 Coronary angiogram (CAG) in LAO cranial view showing normal mid and distal left anterior descending (LAD) artery.
Fig. 8 Coronary angiogram (CAG) in LAO cranial view showing normal mid and distal left anterior descending (LAD) artery.
Discussant
Takotsubo Cardiomyopathy
Normal epicardial coronary arteries in our case establishes the diagnosis of Takotsubo cardiomyopathy. Diagnosis of Takotsubo cardiomyopathy is certain if there is an absence of culprit atherosclerotic coronary artery disease, including acute plaque rupture, thrombus formation, and coronary dissection or other pathologic conditions, to explain the pattern of temporary LV dysfunction observed (e.g., hypertrophic cardiomyopathy, viral myocarditis).16 RWMAs of LV or right ventricle (RV) myocardium occur and are frequently, but not always, preceded by a stressful trigger (emotional or physical).
Emotional triggers are present in our patient because of multiple surgeries for RA and need for another surgery (hysterectomy) in few days.
Acute Anterior Wall MI
Diagnosis of anterior wall AMI is ruled out in our patient because of normal epicardial coronary arteries. RA is known for premature atherosclerosis, but in our patient, there is no evidence of plaque in coronary angiogram, so plaque rupture, erosion and thrombosis are ruled out. There is no luminal irregularities in coronary arteries to suspect coronary arteritis.
Clinical Impression
Takotsubo cardiomyopathy is the diagnosis in our patient because of minimal elevation of cardiac troponins and more elevation in NT pro BNP, associated with normal coronaries (Figs. 7 and 8) in the presence of emotional trigger. Clinical follow-up after 3 months has shown normal LV function in echocardiography, suggesting reversible dysfunction of LV.
Clinical Diagnosis
Takotsubo Cardiomyopathy
Discussion of Management
Takotsubo cardiomyopathy is a self-limited disorder, usually with rapid resolution of the symptoms and LV dysfunction. The patient was treated with β-blockers and angiotensin-converting enzyme (ACE) inhibitors. Follow-up after 3 months has shown improvement in symptoms and LV function. Follow-up 2D echo has shown EF of 52% at 3 months with improvement in functional New York heart Association (NYHA) class from II to III. The European task force position statement suggests classification of patients with Takotsubo cardiomyopathy into lower-risk and higher-risk categories, with the latter based on an LV EF of less than 45%, hypotension and an outflow tract gradient of greater than 40 mm Hg, and/or the presence of an arrhythmia.17 Consideration of an ACE inhibitor and/or a β-blocker is recommended in the higher-risk groups.17 In our patient, EF is 42% at the time of admission, so she comes under high-risk category and was treated with ACE inhibitors and β-blockers. Leading hypothesis suggests that a catecholamine surge results in regional microvascular dysfunction in susceptible patients, accompanied by cellular calcium overload.18
Final Diagnosis
Takotsubo cardiomyopathy.
Conflict of Interest
None declared.
References
- Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350-1358.
- [Google Scholar]
- Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(12):1605-1613.
- [Google Scholar]
- The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(07):897-903.
- [Google Scholar]
- Treatment of pulmonary hypertension in interstitial lung disease: do not throw out the baby with the bath water. Eur Respir J. 2013;41(04):781-783.
- [Google Scholar]
- Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
- [Google Scholar]
- Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015;67(06):1449-1455.
- [Google Scholar]
- Do we need a disease-specific cardiovascular risk calculator for patients with rheumatoid arthritis? Arthritis Rheumatol. 2015;67(08):1990-1994.
- [Google Scholar]
- Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis. 2015;74(06):1118-1123.
- [Google Scholar]
- Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace. 2011;13(06):780-788.
- [Google Scholar]
- Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(01):8-27.
- [Google Scholar]
- Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(03):343-346.
- [Google Scholar]
- Dynamic changes in ST segment resolution after myocardial infarction and the association with microvascular injury on cardiac magnetic resonance imaging. Heart Lung Circ. 2011;20(02):111-118.
- [Google Scholar]
- Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(04):472-479.
- [Google Scholar]
- Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529.
- [Google Scholar]
- Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27(02):192-198.
- [Google Scholar]







