Translate this page into:
A Young Pregnant Woman with Aortic Coarctation and Hypertrophic Cardiomyopathy
*Corresponding author: Balamurugan Natarajan, Department of Cardiology, Kilapuk Medical College and Hospital, Puducherry, India. balambbs08@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Natarajan B, Arumugam MA, Elamaran C. A Young Pregnant Woman with Aortic Coarctation and Hypertrophic Cardiomyopathy. Indian J Cardiovasc Dis Women. doi: 10.25259/ IJCDW_1_2025
Abstract
Pregnancy outcomes are negatively impacted by hypertrophic cardiomyopathy (HCM) and aortic coarctation (CoA), two distinct and independent cardiovascular conditions. Aortic aneurysms, hypertension, ischemic stroke, and re-coarctation are among high rates of cardiac complications seen in patients with repaired CoA. Modified World Health Organization (mWHO) maternal cardiovascular risk classification now categorizes pregnant women with repaired CoA in mWHO class III, whereas those with unrepaired severe CoA have been classified in mWHO class IV. Patients with HCM are categorized as mWHO II–III based on whether they have left ventricular outflow obstruction.
Keywords
Cardiac magnetic resonance imaging
Coarctation of the aorta
Hypertrophic cardiomyopathy
Left ventricular outflow tract
World Health Organization
INTRODUCTION
Aortic coarctation (CoA), a congenital heart disease (CHD), is marked by a ridge-like focal narrowing of the aortic lumen caused by thickening of the medial wall and infolding of the aortic wall tissue.[1-3] Non-physiological left ventricular (LV) hypertrophy is a characteristic of “Hypertrophic Cardiomyopathy (HCM),” an inherited heart condition brought on by abnormalities in the genes encoding cardiac sarcomere proteins.[4] CoA can appear alone or in conjunction with the transposition of the great arteries, bicuspid aortic valve, “patent ductus arteriosus (PDA), atrial septal defect (ASD), ventricular septal defect (VSD),” or left-sided obstructive lesions that involve aortic, subaortic, as well as mitral stenosis.[5] There are no reports in literature of CoA associated with hypertrophic cardiomyopathy (HCM) during pregnancy. In this case, HCM and CoA occurred simultaneously during the initial trimester of pregnancy. The severity of these two conditions is linked to negative maternal and fetal outcomes. In addition, we discuss the challenges in imaging and managing this patient during pregnancy.
CASE REPORT
A 27-year-old primipara arrived at our hospital at 11 weeks of pregnancy with complaints of exertional dyspnea for the past 2 months. In her early years, the patient was diagnosed with CoA. In 2010, she had shunt surgery between the “descending aorta and the left subclavian artery. A patent shunt between the left subclavian artery and descending aorta was discovered” during a cardiac computed tomography (CT) scan 3 years later after the patient began having trouble breathing when exerting themselves [Figures 1 and 2]. In addition, a perfectly functioning surgical shunt was found during a cardiac catheterization. After using amlodipine and enalapril for 3 years, the patient stopped taking them and was no longer monitored. She had no family history of sudden cardiac death, CHD, or consanguineous marriage. The obstetric department recommended a heart screening for the patient, who is presently in her first trimester of pregnancy. Clinical examination revealed a persistent murmur in the interscapular region and an “ejection systolic murmur in the left parasternal area. The lower limb’s blood pressure was 122/82 mmHg, and the upper limbs were 140/90 mmHg. The patient’s Echocardiography (ECG) revealed LV hypertrophy with a strain pattern along with a normal sinus rhythm. The results of routine blood testing were within normal limits. With systolic anterior motion of the mitral leaflet, transthoracic echocardiography (TTE) revealed marked hypertrophy of the anterior interventricular septum (25 mm), posterior interventricular septum (23 mm), LV anterior wall (16 mm), LV lateral wall (17 mm), and LV posterior wall (13 mm). In addition, abnormal papillary muscle insertion into the LV apex caused mid-LV obstruction, with an LV mid-cavity gradient of 67 mmHg [Figures 3 and 4]. These findings suggest HCM-obstructive type, with absent LV outflow tract gradient. It was difficult to observe the surgical shunt that ran across the descending aorta and left subclavian artery.” According to the Doppler study, the peak systolic gradient across the coarctation was 71 mmHg, and the peak velocity was 4.2 m/s. A sawtooth pattern with diastolic outflow was displayed by continuous Doppler [Figure 5]. With a low systolic amplitude and continuous diastolic flow, the descending aorta measured around 11 mm [Figure 6]. The carotid Doppler, fundus examination, and lower limb Doppler were all normal; however, the ultrasonography abdomen showed a single live intrauterine gestation. A cardiac magnetic resonance imaging (MRI) revealed shunt failure, with the descending and ascending aortas measuring 1.3 and 2.7 cm, respectively [Figure 7]. In addition, there was aberrant papillary muscle attachment to the LV apex and a hypertrophied interventricular septum with a diastolic septal thickness of 2.5 cm [Figure 8]. Following confirmation of the diagnosis of severe CoA with HCM, medical termination of pregnancy was recommended. Despite counsel, the patient chose to carry on with the pregnancy, and 2 weeks later, pulmonary edema and severe breathing problems were the result of accelerated hypertension. Following supportive care and an emergency medical pregnancy termination, the patient made a full recovery.
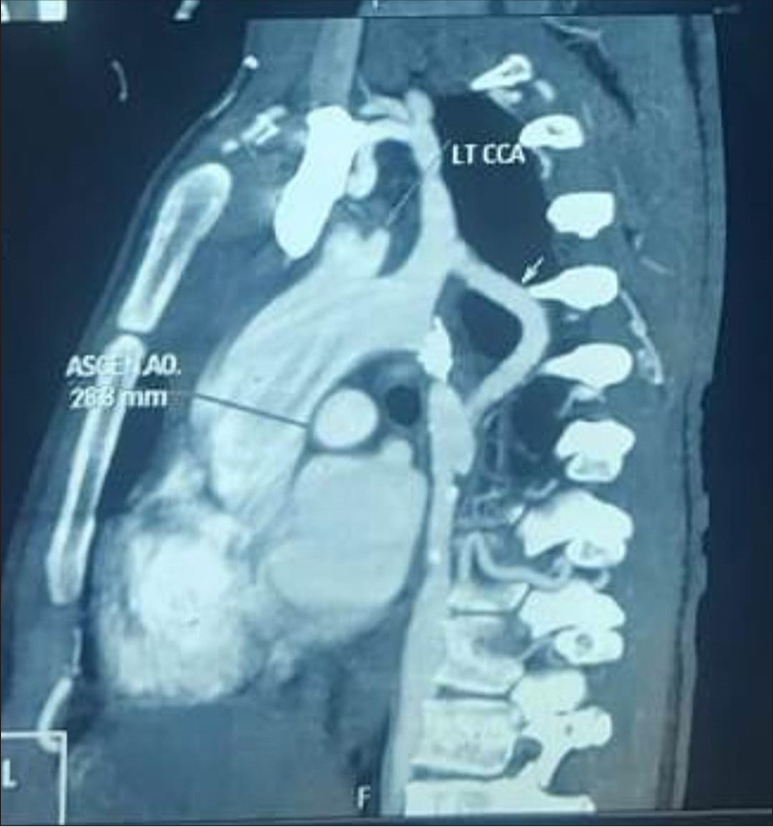
- Cardiac computed tomography scan showing a functioning shunt between the left subclavian artery and descending aorta. (LTCCA: Left common carotid artery, ASCEN.AO: Ascending aorta, AO: Aorta.)
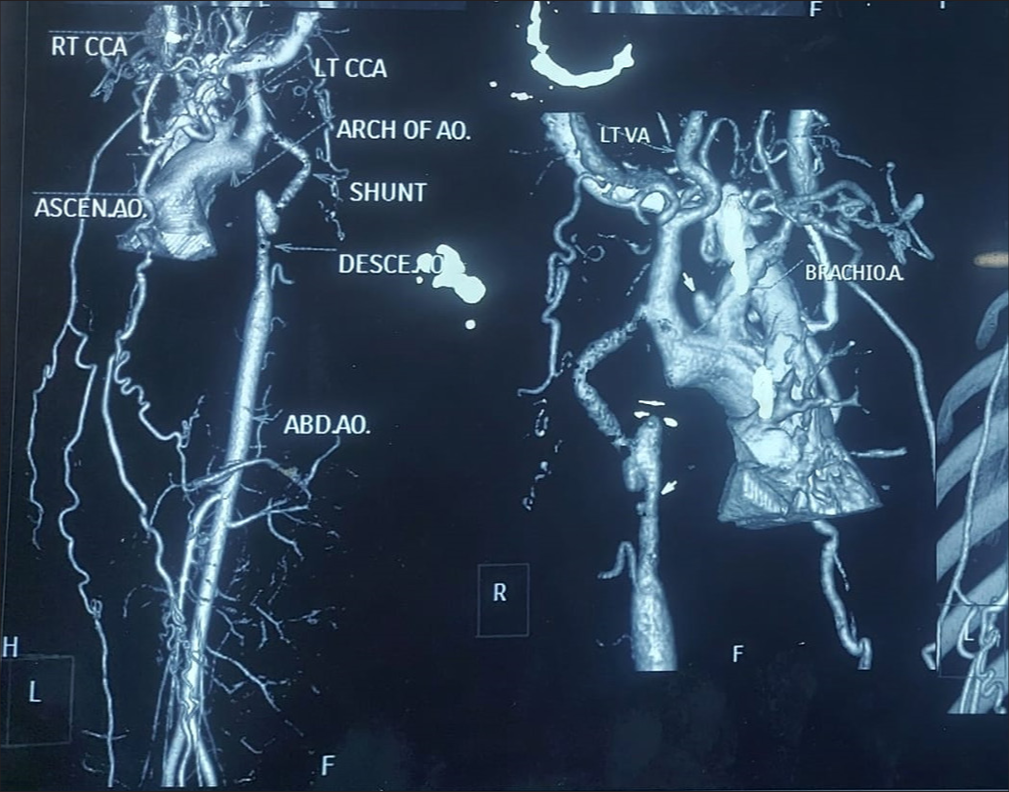
- Computed tomography aortogram. (LTCCA: Left common carotid artery, ASCEN.AO: Ascending aorta, AO: Aorta, DESCE..AO: Descending aorta, ABD.AO: Abdominal aorta, VA: Vertebral artery, BRACHIOA: Brachiocephalic trunk.)

- 2D ECHO showing asymmetrical hypertrophy with abnormal attachment of papillary muscle. (ECHO: Echocardiography.)
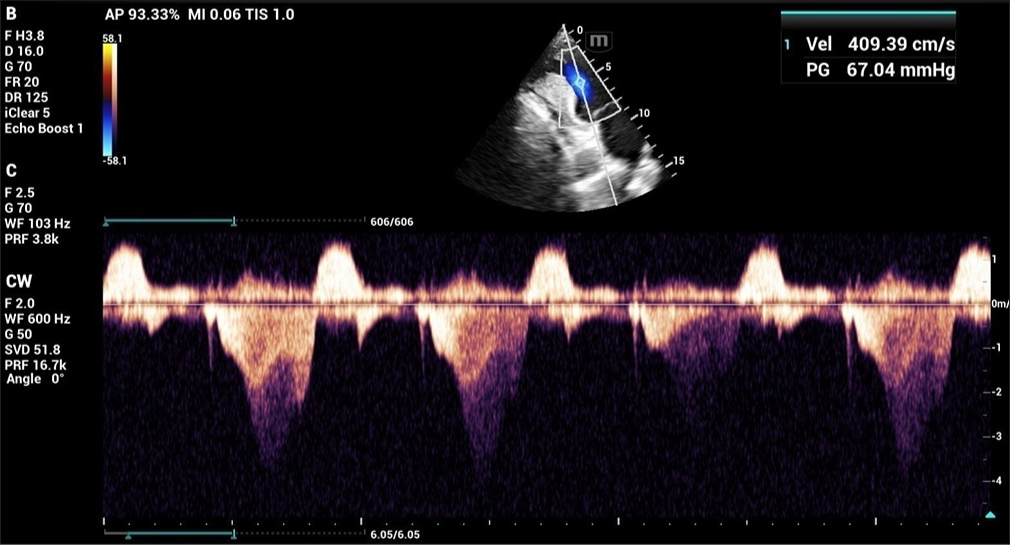
- 2D ECHO showing mid-left ventricular gradient. (ECHO: Echocardiography.)
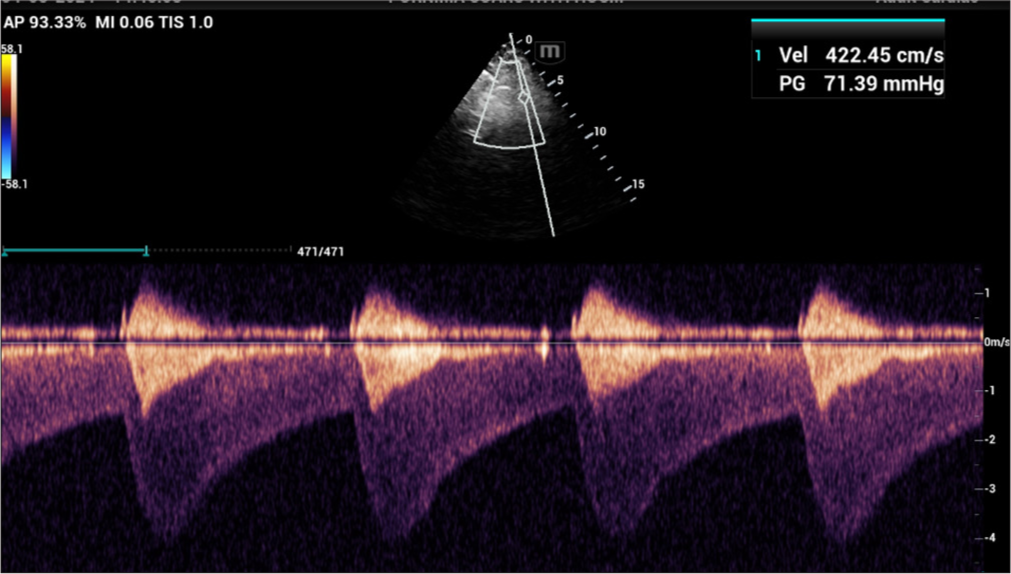
- Continuous Doppler showed a sawtooth pattern with diastolic runoff.
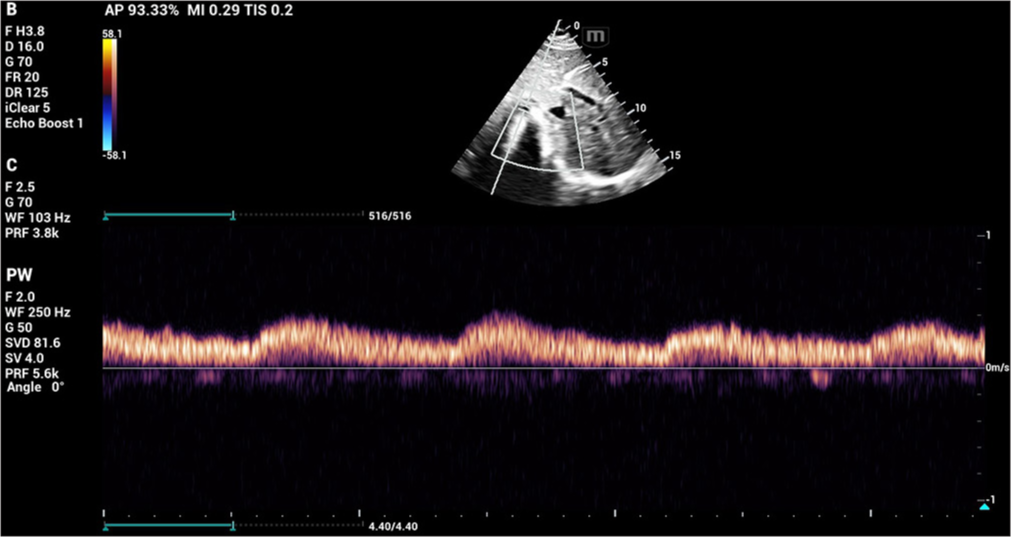
- The descending aorta measured about 11 mm with low systolic amplitude and continuous flow in diastole.
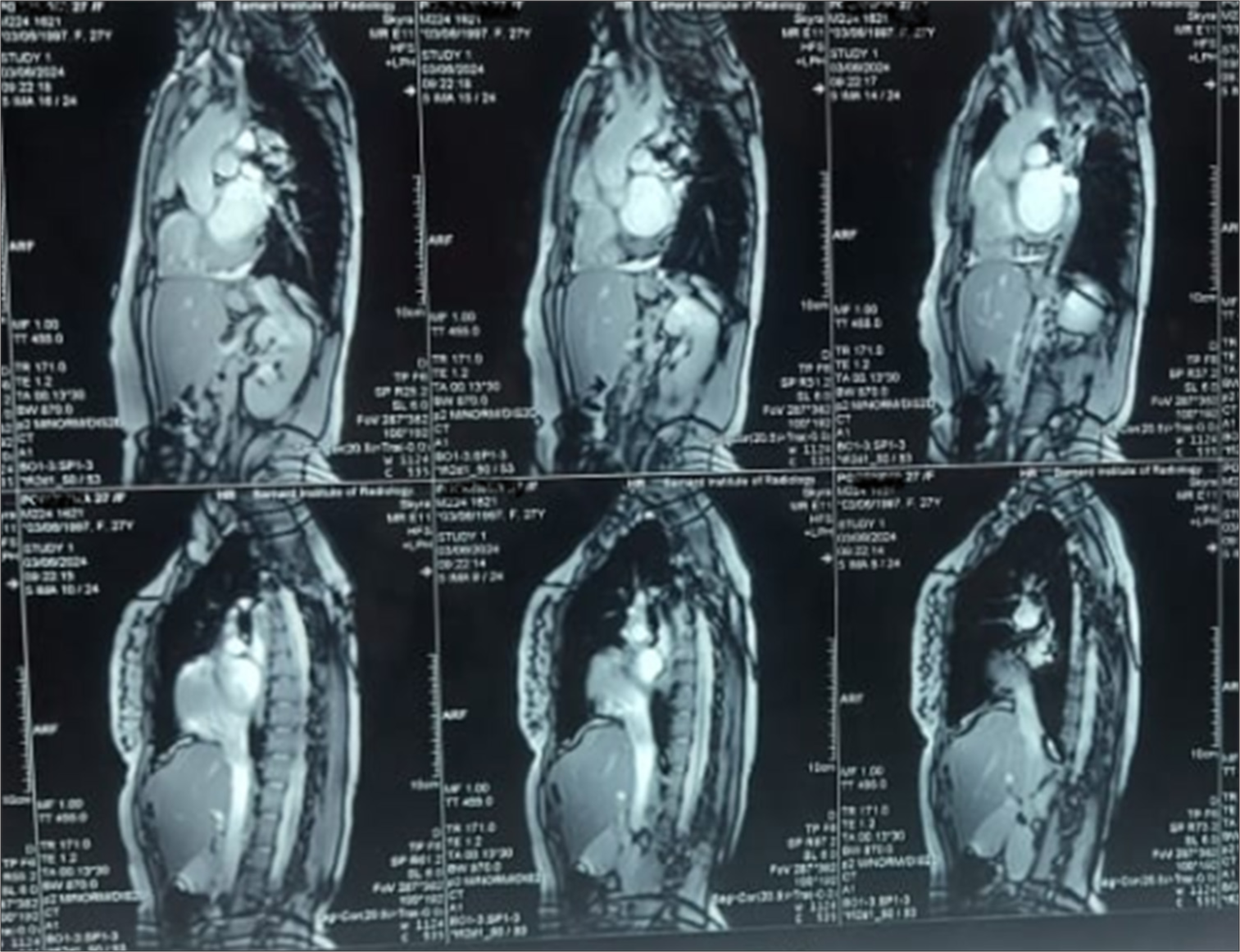
- Cardiac magnetic resonance imaging showing aortic coarctation.
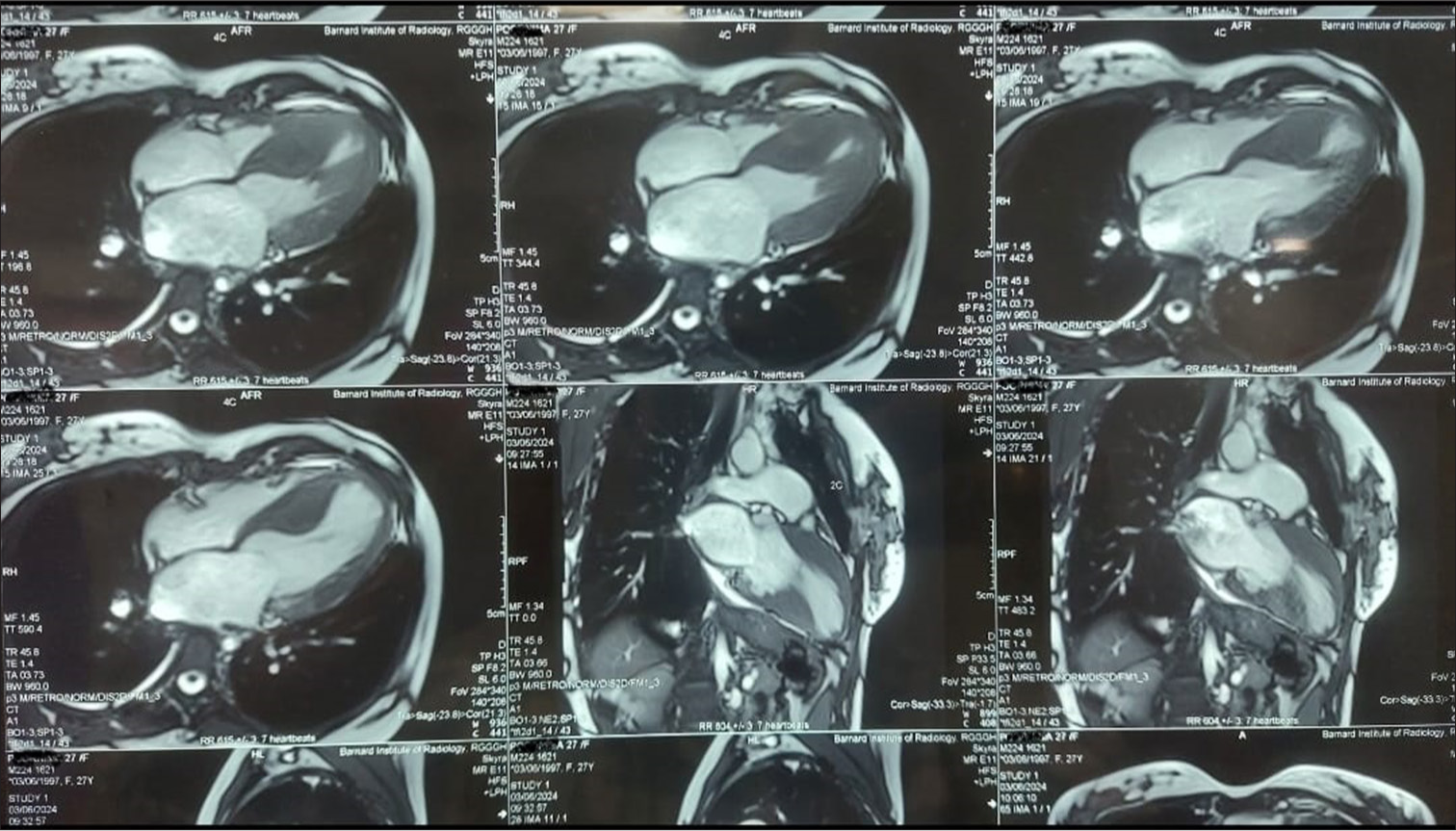
- Cardiac magnetic resonance imaging showing a hypertrophied interventricular septum with a diastolic septal thickness of 2.5 cm, along with abnormal papillary muscle attachment to the left ventricular apex.
DISCUSSION
The fifth most prevalent congenital cardiovascular defect is CoA. It usually demonstrates as discrete stenosis of aortic isthmus and encompasses a range of aortic narrowing from tubular hypoplasia to isolated stenosis of thoracic aorta.[6] In contrast “to right-sided lesions, left-sided cardiac obstructions are more common in men than in women, and the majority of afflicted individuals are men.[7,8] HCM is an autosomal dominant disorder” resulting from abnormalities in genes that encode cardiac sarcomeres.[9] It is recognized when one or more segments of the LV myocardium demonstrate an increase in wall thickness, often exceeding 15 mm, following the exclusion of other cardiac or systemic conditions.[10] HCM is often well tolerated during gestation, with most patients classified as class II as per the modified “World Health Organization” and patients with significant “Left Ventricular Outflow Tract Obstruction” are at elevated risk during pregnancy and are categorized as class III–IV, based on severity.[11] Bicuspid aortic valve, ASD, VSD, PDA, “transposition of great arteries, or left-sided obstructive lesions” that include mitral, aortic, and subaortic stenosis may develop with CoA.[5] Untreated, CoA is a prevalent congenital heart condition with a poor prognosis.[12] Repairing CoA improves long-term survival but increases risk of cardiac issues that include “re-34% (coarctation), 18% (aortic aneurysms), 32% (hypertension), and ischemic stroke 13-fold.[13,14] Women with unrepaired severe CoA are mWHO IV. Repaired CoA in pregnant women is classed as mWHO II–III, which indicates an intermediate risk of maternal mortality and moderate-to-severe morbidity.”[15] Clinically, these two CHDs are irrelevant and rarely coexist. This patient was diagnosed with CoA in childhood that was corrected with shunt placement; later, the patient lost follow-up. At present, a patient came for cardiac screening in the first trimester of pregnancy and was diagnosed to have recoarctation of the aortic shunt with coexisting HCM. CT contrast imaging and invasive catheterization were contraindicated as the patient was in the first trimester of pregnancy, and the diagnosis was made with 2D TTE and MRI imaging. Echocardiography and MRI are safe during pregnancy and effectively assess restenosis.[16] This patient exhibited severe CoA as demonstrated by 2D TTE data, indicating “peak velocity of 4.2 m/s, a peak systolic gradient of 71 mmHg, diastolic runoff, and continuous flow in the abdominal aorta. Asymmetric septal hypertrophy, characterized by a septal to posterior wall thickness ratio exceeding” 1.5, signifies concomitant HCM. Cardiac MRI revealed descending and ascending aorta dimensions of 1.3 cm and 2.7 cm, respectively, with flow velocity imaging indicating no flow across the shunt. In addition, there was a hypertrophied interventricular septum with a diastolic septal thickness of 2.5 cm and abnormal papillary muscle attachment to LV apex. Diagnosis of severe coarctation of aorta with HCM has been confirmed, and the patient has been advised to terminate the pregnancy. Still, the patient was not willing to undergo medical termination of pregnancy. Two weeks later, the patient developed an acute pulmonary edema with hypertensive crisis, necessitating an immediate medical termination of pregnancy. Post-termination of pregnancy and with supportive treatment, the patient’s symptoms improved clinically. Yang et al. stated a case of CoA and HCM in a non-pregnant 35-year-old woman with a history of hypertension and syncope.[17] A defining characteristic of HCM is asymmetric hypertrophy, which can be identified by “a septal-to-posterior wall thickness ratio of ≥1.3 in normotensive individuals or ≥1.5 in hypertensive individuals.” Nevertheless, certain hypertensive patients exhibit it.[18] A prevalent surrogate marker for determining the severity of CoA is Doppler echocardiographic systolic peak gradient.[19,20] It has a good correlation with other noninvasive techniques that include cardiac MRI,[21] arm-leg systolic pressure difference,[20] and invasiveness with pressure gradient measured by catheterization.[19] Pregnant women with CoA are more likely to develop hypertension-related complications and have poor cardiovascular outcomes.[18] An integrated team approach is required for the management of these patients who are considering pregnancy, and such individuals must undergo preconception screening and counseling.
CONCLUSION
This patient had undergone CoA repair with a shunt between LSA and descending thoracic aorta in childhood; however, they received no follow-up. As recommended by an obstetrician, the patient sought evaluation from a cardiologist because she was pregnant. The patient had a recurrence of CoA due to shunt failure with associated HCM. Catastrophic disasters have been prevented as a result of prompt assessment and action. In conclusion, HCM may be associated with conditions like “bicuspid aortic valve and coarctation of aorta.” Pregnancy significantly increases the risk of long-term problems for patients with CoA, which include berry aneurysm, shunt restenosis, persistent hypertension, and aortic dissection. Preconception counseling and a shunt status check should be provided to patients who are considering pregnancy. The most effective techniques for identifying CoA and restenosis during pregnancy are echocardiography and MRI. An obstetrician and a cardiologist must function as a multidisciplinary team to manage these patients due to the elevated risk of fetal and maternal issues.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- CT and MRI of Aortic Coarctation: Pre-and Postsurgical Findings. AJR Am J Roentgenol. 2015;204:W224-33.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogeneity of Ventricular Repolarization in Newborns with Severe Aortic Coarctation. Pediatr Cardiol. 2012;33:302-6.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy, Image Quality, and Radiation Dose of Prospectively ECG-Triggered High-Pitch Dual-Source CT Angiography in Infants and Children with Complex Coarctation of the Aorta. Acad Radiol. 2014;21:1248-54.
- [CrossRef] [PubMed] [Google Scholar]
- 2014 ESC Guidelines on Diagnosis and Management of Hypertrophic Cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733-79.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, Morphology, and Progression of Bicuspid Aortic Valve in Pediatric and Young Adult Subjects with Coexisting Congenital Heart Defects. Congenit Heart Dis. 2017;12:261-9.
- [CrossRef] [PubMed] [Google Scholar]
- Coarctation of the Aorta: From Fetal Life to Adulthood. Cardiol J. 2011;18:487-95.
- [CrossRef] [PubMed] [Google Scholar]
- The Aetiology of Coarctation of the Aorta. Lancet. 1961;1:463-8.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of Congenital Heart Disease: II. Prenatal Incidence. Pediatr Cardiol. 1995;16:155-65.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Hypertrophic Cardiomyopathy in a General Population of Young Adults. Echocardiographic Analysis of 4111 Subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785-9.
- [CrossRef] [PubMed] [Google Scholar]
- 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in Collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212-60.
- [Google Scholar]
- Hypertrophic Cardiomyopathy and Pregnancy: A Retrospective Analysis from a Tertiary Care Hospital. Tex Heart Inst J. 2022;49:e207427.
- [CrossRef] [PubMed] [Google Scholar]
- Coarctation of the Aorta: Natural History and Outcome after Surgical Treatment. QJM. 1999;92:365-71.
- [CrossRef] [PubMed] [Google Scholar]
- Late Outcomes in Adults with Coarctation of the Aorta. Heart. 2015;101:1190-5.
- [CrossRef] [PubMed] [Google Scholar]
- Coarctation of the Aorta: Lifelong Surveillance is Mandatory Following Surgical Repair. J Am Coll Cardiol. 2013;62:1020-5.
- [CrossRef] [PubMed] [Google Scholar]
- 2018 ESC Guidelines for the Management of Cardiovascular Diseases During Pregnancy. Eur Heart J. 2018;39:3165-241.
- [CrossRef] [Google Scholar]
- Outcome of Pregnancy Following Intervention for Coarctation of the Aorta. Am J Cardiol. 1998;82:786-8.
- [CrossRef] [PubMed] [Google Scholar]
- Aortic Coarctation Associated with Hypertrophic Cardiomyopathy in a Woman with Hypertension and Syncope: A Case Report with 8-Year Follow-Up. Front Cardiovasc Med. 2021;8:818884.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Multimodality Cardiac Imaging in the Management of Patients with Hypertrophic Cardiomyopathy: An Expert Consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16:280.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy and Pitfalls of Doppler Evaluation of the Pressure Gradient in Aortic Coarctation. J Am Coll Cardiol. 1986;7:1379-85.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Continuous Wave Doppler Ultrasound Velocimetry to Assess the Severity of Coarctation of the Aorta by Measurement of Aortic Flow Velocities. Br Heart J. 1984;52:278-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Risk of Hypertensive Complications of Pregnancy among Women with Versus without Coarctation of the Aorta. Am J Cardiol. 2011;107:1529-34.
- [CrossRef] [PubMed] [Google Scholar]







