Translate this page into:
A Study to Assess the Acceptance of Doctors toward the Practice of Reusing of Cardiac Disposables in Coronary Angioplasty in a Tertiary Care Hospital
*Corresponding author: Pushpi Rani, Department of Hospital Administration, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India .pushpi.sinha23@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rani P, Irshad H, Rashid S, Nimma S. A study to assess the acceptance of doctors toward the practice of reusing of cardiac disposables in coronary angioplasty in a tertiary care hospital. Indian J Cardiovasc Dis Women. 2023;8:223-9.
Abstract
Objectives:
Health-care providers are expected to deliver quality patient care cost-effectively, especially in developing countries. Medical devices labeled “single use only” should ideally not be reused but, can be considered for reuse due to their higher cost. Stringently regulated reprocessing of the single-use device provides an opportunity to do so along with the potential to have a favorable impact on environmental waste. The aim of this study is to assess the acceptance of doctors toward the practice of reusing cardiac disposables in coronary angioplasty.
Materials and Methods:
An open-ended and self-designed questionnaire was prepared and was standardized by a panel of cardiologists to assess doctors’ acceptance toward reusing cardiac disposable in coronary angioplasty. The cardiologists were interviewed individually, and the responses were analyzed.
Results:
The cardiologists were aware that the angioplasty disposables were being reused. The majority were satisfied with the sterilization of disposables and were of the opinion that it was safe for the patients. Cardiac catheters were reused 5–6 times while the balloons were reused 3–4 times. An increase in procedure time of 10–15 min was observed in 30% of the patients. There was an increase, in contrast, volume of about 40–50 mL with reused cardiac catheters and balloons which was observed in 15% of the patients. Defect in the physical integrity of the catheter, that is, tip abrasion/microfracture was observed in 1% of the patients. Deflation dysfunction of the balloon during the procedure was observed in 2% of the patients, while breakage of the balloon from the shaft and entanglement of the balloon was observed in 1% of the patients. Balloon rupture during angioplasty was observed in 8% of the patients. Around 1.5% of the patients undergoing angioplasty with reused disposables were presented with infections. Thrombosis was observed in 3% of the patients with reused disposables, and it was the most common adverse event post-angioplasty. There were cases where the patients were sent for emergency surgery/coronary artery bypass graft surgery due to balloon entanglement and about 0.1% of the patients were found to have such a complication.
Conclusion:
Cardiac disposables can be safely reused by health-care professionals, especially in developing nations due to their budget constraints, provided it is reused no more than 3–5 times and efficient methods of sterilization are observed.
Keywords
Reuse
Single-use devices
Cardiac disposables
Balloons
Cardiac catheters
ABSTRACT IMAGE

INTRODUCTION
Health-care providers are expected to deliver quality patient care cost-effectively, especially in developing countries. Medical devices labeled “single use only” should ideally not be reused but, can be considered for reuse due to their higher cost. Stringently regulated reprocessing of the single-use device (SUD) provides an opportunity to do so along with the potential to have a favorable impact on environmental waste. Hospitals have been reusing SUDs since the 1970s.[1,2] Cost saving on medical expenditure is one of the important reasons for reprocessing SUDs. In the US, annual estimated savings with reprocessing of SUDs have been reported to be $1.8 billion approximately.[3] Cost estimate studies conducted in Germany have reported savings of 20 million Euros yearly from reprocessing angioplasty balloon catheters.[4] Apart from cost savings, reprocessing is also considered as an optimal strategy to reduce the environmental footprint of a hospital. Therefore, reprocessing has been identified as a Smarter Purchasing Challenge in the Healthier Hospitals Initiative.[5]
The manufacturer labels a device as single-use, either due to the safety and reliability concerns or because the manufacturer chooses not to conduct the studies needed to prove otherwise.[6] Since the Food and Drug Administration (FDA) can only evaluate a device for its intended use provided by the manufacturer, it only implies it can be safely and reliably used once. However, it does not imply that it cannot be reused safely and reliably if reprocessed appropriately. Moreover, manufacturers oftentimes change labels from reusable to single-use, without any significant change in material, design, or performance and such a change in labeling does not usually require approval from the FDA; which does not even mandate any device to be labeled single-use.[7] Original equipment manufacturers market devices as single-use when they could just as well be reusable was driven by economic incentives. This created apprehension in the minds of health-care professionals. Occasionally, many manufacturers themselves offer their own recycling and reprocessing programs, further questioning the relevance of “single-use” designation and the necessity of complying with it.[8] The mechanical performance of catheters sometimes fails with reports of failure to cross tight coronary lesions, longer procedure times, and use of a higher volume of contrast. Hospitals opting to reuse these catheters need to ensure safety, both in terms of sterilization and performance, and not reuse luminal catheters more than 3 times.[9]
Limited literature is available regarding the safety and acceptance of the reuse of disposables by health-care professionals in India. Therefore, an interview-based study was conducted to assess doctors’ acceptance toward reusing cardiac disposables in coronary angioplasty.
MATERIALS AND METHODS
An open-ended and self-designed questionnaire [Table 1] was prepared and standardized by a panel of cardiologists to assess doctors’ acceptance toward reusing cardiac disposables in coronary angioplasty. The cardiologists were interviewed individually and the responses were analyzed.
| Questions |
|---|
| Are you aware that the disposables are being reused for angioplasty in your hospital? |
| Are you satisfied with the sterilization of the disposables for angioplasty? |
| Do you think the reused catheters are safe for the patient? |
| How many times do you reuse cardiac catheters and balloons for angioplasty? |
| Was there any increase in procedure duration with reused cardiac catheters? |
| Was there any increase in the volume of contrast used for Angioplasty with reused cardiac catheters and balloons? |
| Was there any issue with physical integrity of the cardiac catheter? |
| Was there any issue with physical integrity of the angioplasty balloons? |
| Was there any incident of angioplasty balloon rupture with reused balloon inflation device? |
| Were there any cases of HIV and Hepatitis transmission with reused disposables? |
| Were the patients undergoing Angioplasty with reused disposables more prone to infections postoperatively compared to the patient undergoing procedure with new disposables? |
| Was there any adverse event following Angioplasty with reused disposables? |
| Was there any increase in thrombosis formation with reused cardiac catheter and balloon? |
| Were there any patients who were sent for emergency surgery/CABG due to complication with reused cardiac catheter and balloon? If yes, what was the complication and how frequently such cases were observed? |
| Was there any case with Ethylene oxide toxicity (ETO sterilization)? |
CABG: coronary artery bypass graft
RESULTS
All the cardiologists in the study were aware that the angioplasty disposables were being reused. The majority (85.7%) of the cardiologists were satisfied with the sterilization of disposables and were of the opinion that it was safe for the patients. It was observed that cardiac catheters were reused 5–6 times while the balloons were reused 3–4 times [Table 2].
| Questions | Result |
|---|---|
| Are you aware that the disposables are being reused for angioplasty in your hospital? | Yes |
| Are you satisfied with the sterilization of the disposables for angioplasty? | Yes |
| Do you think the reused catheters are safe for the patient? | Yes |
| How many times do you reuse cardiac catheters and balloons for angioplasty? | Catheter: 5–6 times Balloon: 3–4 times |
| Was there any increase in procedure duration with reused cardiac catheters? | 30% patients; delay of 10–15 min |
| Was there any increase in the volume of contrast used for Angioplasty with reused cardiac catheters and balloons? | 15% patients, increase of 40–50 mL |
| Was there any issue with physical integrity of the cardiac catheter? | Tip abrasion/Microfracture: 1% |
| Was there any issue with physical integrity of the angioplasty balloons? | Deflation dysfunction: 2% Breakage and Entanglement: 1% |
| Was there any incident of angioplasty balloon rupture with reused balloon inflation device? | 8% Patients |
| Were there any cases of HIV and hepatitis transmission with reused disposables? | No |
| Were the patients undergoing Angioplasty with reused disposables more prone to infections postoperatively compared to the patient undergoing procedure with new disposables? | 1.5% Patients |
| Was there any adverse event following Angioplasty with reused disposables? | Thrombosis |
| Was there any increase in thrombosis formation with reused cardiac catheter and balloon? | 3% Patients |
| Were there any patients who were sent for emergency surgery/CABG due to complication with reused cardiac catheter and balloon? If yes, what was the complication and how frequently such cases were observed? | 0.1% Patients due to Entanglement of balloon |
| Was there any case with Ethylene oxide toxicity (ETO sterilization)? | No |
CABG: coronary artery bypass graft
Procedure time
An increase in procedure time of 10–15 min was observed in 30% of patients. Furthermore, an increase in procedure time of more than 20 min increased heparin dosage [Figure 1].
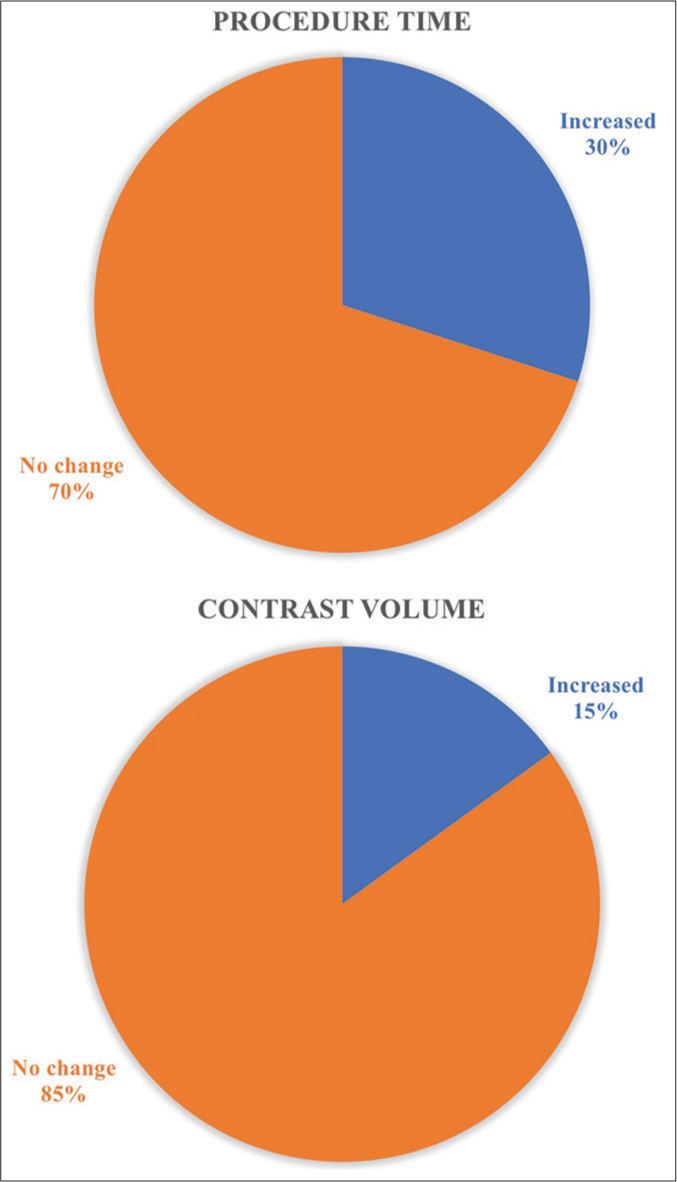
- The increase in procedure time and contrast volume with reused disposables.
Contrast volume
An increase in contrast volume of about 40-50 mL was observed in 15% of patients with reused cardiac catheters and balloons [Figure 1].
Physical integrity of the catheter
Tip abrasion/microfracture was observed in 1% of patients with a reused cardiac catheter. Deflation dysfunction of the balloon during the procedure was observed in 2% of patients, while breakage of the balloon from the shaft and entanglement of the balloon was observed in 1% of patients. Balloon rupture during angioplasty was observed in 8% of patients [Figure 2].
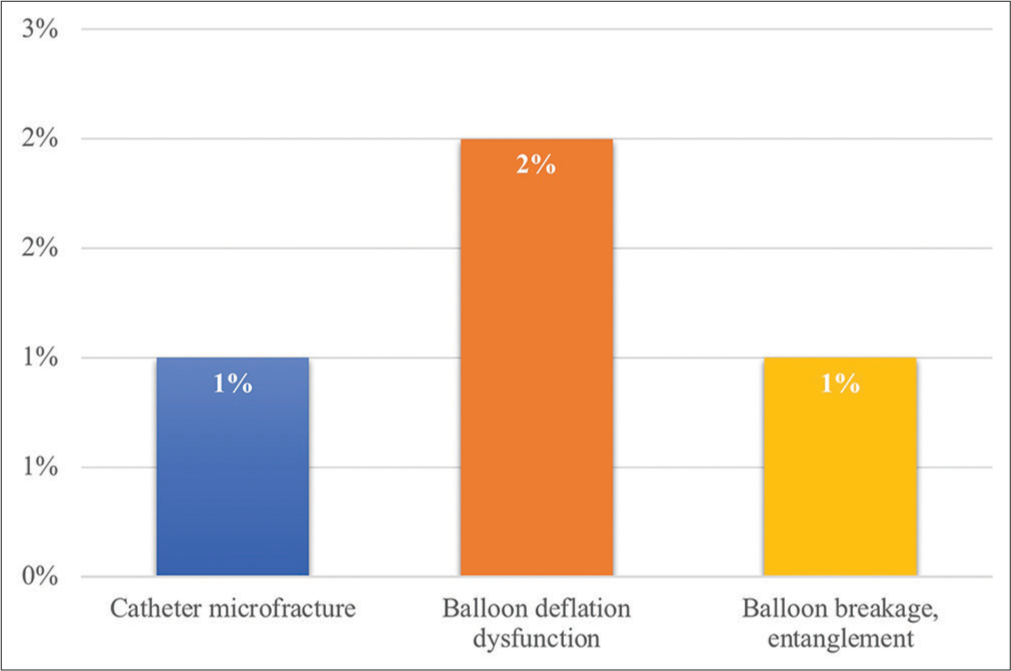
- The issues with the physical integrity of the reused cardiac catheter and balloon.
Incidence of infection
Around 1.5% of patients undergoing angioplasty with reused disposables presented with infections. Cases of HIV and Hepatitis post-angioplasty were extremely rare.
Adverse events
Adverse events following angioplasty included thrombosis, myocardial infarction, coronary artery bypass graft surgery (CABG), and death. Thrombosis was observed in 3% of patients with reused disposables, and it was the most common adverse event post-angioplasty. Around 1% of the patient who underwent angioplasty with reused disposables developed myocardial infarction. There were cases where the patients were sent for emergency surgery/CABG due to balloon entanglement and about 0.1% of patients were found to have a such complication. Moreover, no mortality was reported with reused cardiac disposables [Figure 3].

- The adverse events following angioplasty with reused cardiac disposables.
There was no case of ethylene oxide toxicity observed with reused disposables.
DISCUSSION
In a study conducted by Frank et al.,[10] in 1988, it was observed that 4.7% of patients developed fever when the catheter was reused once or twice and 6% of the patients developed fever when reused more than twice. Whereas, as per our questionnaire, it was found that 1.5% of the patients developed infection post-percutaneous transluminal coronary angioplasty (PTCA) with reused consumables which may be explained by the advances in the technology of sterilization over a period.
As per our questionnaire based study, the procedure time of angioplasty increased by 10-15 minutes. Similar results were found in a study conducted by Mak et al.,[11] in 1996 and they concluded that the procedure time was increased by 13 min.
Moreover, Mak et al.[11] observed that the contrast volume was increased by 36 mL in patients with reused disposables which was similar to the present study. Their study also found that adverse events such as myocardial infarction, emergency surgery (CABG), and death were observed in 3.1%, 5.6%, and 1.9%, respectively. On the contrary, as per our questionnaire, 1% of patients presented with myocardial infarction, 0.1% of patients underwent CABG due to entanglement of the balloon, and no mortality with reused cardiac consumables.
In a study conducted by Habib et al.,[12] in 2019, 135 cardiac disposables were collected, of which 90 were sterilized by exposure to formaldehyde and had negative cultures, while the rest 45 disposables that were sterilized with cidex, staphylococcus were isolated from three samples (P = 0.016). There was no episode of catheter fracture or pyrogen reactions. Cost analysis concluded a saving of approximately 5000 and 11,500 dollars/100 procedures with an average of five uses for diagnostic disposables and three uses for angioplasty disposables, respectively. While, according to our questionnaire, 1.5% of the patients developed pyrogenic reaction post-PTCA with reused disposables which may be explained by the advances in the technology of sterilization over a period. Furthermore, catheter tip abrasion/ microfracture was observed in 1% of patients and breakage of the balloon from the shaft was observed in 1% of patients.
In a randomized study conducted by Zubaid et al in 2001, clinical and angiographic success of reused versus new coronary angioplasty balloon catheters were compared across 377 procedures. The incidence of first balloon failure with reused catheter (12/178 cases, 7%) was similar to that of new catheter (10/199 cases, 5%). Also, angiographic success rates were also similar in bot, that is, 98.9% and 98.5%, respectively. The number of balloon catheters used per lesion, volume of contrast injected and procedural and fluoroscopy time were similar in both reused and new catheters. The incidence of major adverse events was similar in both, that is, 8 cases (4.5%) with the reused catheter and 10 cases (5%) with the new catheter. The study concluded that, the results of reused balloon catheters were similar to that of new catheters with more cost saving per procedure with reused balloon catheters.[3]
In India, there are no third-party reprocessors of single use devices; however, in-house reprocessing is done in most hospitals. A survey was conducted in 1997 across 26 coronary angioplasty centers, which had been practicing reuse, under the aegis of the Cardiological Society of India. In its draft guidelines for reuse, the committee recommended that the reuse of disposables should be allowed strictly adhering to sterilization norms and all the equipment for reuse should be tested for functional and mechanical integrity. The date of sterilization should be mentioned on the package and re-sterilization should be practiced if any sterilized equipment is not used within 6 months.[13]
Due to the increased unregulated reuse, the FDA in 1999 sought feedback from health-care professionals, manufacturers, and reprocessing firms to determine whether federal oversight was needed to address the issue of reprocessing. The United States (US) Government Accountability Office (GAO) was asked to review the practice of single use device reprocessing in US hospitals and a report entitled “Single-Use Medical Devices: Little Available Evidence of Harm From Reuse, but Oversight Warranted” was submitted in June 2000. The report showed that 20–30% of US hospitals confirmed the reuse of at least one type of single use device and one-third of the hospitals had operational contracts with third-party reprocessors. It is also likely that some hospitals do not report that they reprocess single use devices. However, among cardiovascular products, except electrophysiology catheters, most hospitals in the US and the western world follow a no-reuse policy. The report also stated that to successfully reprocess a device, health-care facilities should stringently follow the standard steps of cleaning, and an inspection of functional integrity and sterilization.[14] US FDA developed strict regulations to monitor reprocessing and quality-controlled evaluation whereby hospitals and third-party reprocessors are subjected to stringent regulations and thereby ensuring the safety and effectiveness of the reprocessed single use devices.[6,15,16]
US GAO report in 2008 concluded that the available evidence indicated no additional health risk from reprocessing single use device. A reprocessed device that meets all the requisite requirements of the Federal Food, Drug, and Cosmetic Act is lawful and may be marketed legally in the US.[17]
Various studies have concluded reprocessing and reuse of coronary angioplasty balloon catheters,[18-25] diagnostic and radio-frequency ablation EP catheters,[26-30] and pacemakers and implantable devices are safe and cost-effective.
Limitations
As the study was questionnaire-based, the exact values of the parameters could not be estimated.
The study had recall bias as the responses were based on their recollection and exposure.
The study did not include a cost-benefit analysis.
CONCLUSION
With advancement in medical science and technology, there has been significant improvement in quality of life and level of care. This progress comes at a high price and may not be economically sustainable, therefore pressurizing health-care professionals to reconcile a limited budget. In such a scenario, the reuse of cardiac disposables is feasible and can be considered a safe strategy by health-care professionals, especially in developing nations, provided it is reused no more than 3–5 times and efficient methods of sterilization are observed.
Suggestions
Every hospital committed to the reuse of SUD should have an institution-specific policy and work with clear guidelines to ensure the safety of patients taking into consideration ethical, regulatory, and legal implications.
There should be a reprocess/reuse committee consisting of doctors, infection control officers/microbiologists, nurses, and administrators to monitor central reprocessing, infection control and cost accounting. The committee should be responsible for devising protocols and managing safety issues.
Standard and validated written protocols should be followed for reprocessing each single use device type. Periodic review and audit to be done.
A record of adverse events should be maintained for all reused devices and there should be a periodic review and audit.
Third-party reprocessing units should be promoted, regulated stringently, and held accountable for quality control.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Audio summary available at:
Financial support and sponsorship
Nil.
References
- Reuse of single-use devices: Looking back, looking forward. Natl Med J India. 2012;25:151-5.
- [Google Scholar]
- A randomized study of the safety and efficacy of reused angioplasty balloon catheters. Indian Heart J. 2001;53:167-71.
- [Google Scholar]
- Reuse of single-use medical devices after quality assured reprocessing: Hygienic, legal and economic aspects, Potential for cost savings in interventional cardiology. Z Kardiol. 2002;91:889-98.
- [CrossRef] [PubMed] [Google Scholar]
- Green your supply chain: 10- steep guide to environmentally preferable purchasing. A practice Greenhealth member publication. Available from : https://practicegreenhealth.org/topics/sustainable-procurement/sustainable-procurement
- [Google Scholar]
- U.S. Department of Health and Human Services. 2008. Food and Drug Administration, Center for Devices and Radiological Health, Guidance for Industry and for FDA Staff: Enforcement Priorities for Single-Use Devices Reprocessed by Third Parties and Hospitals. Available from: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm107172.pdf
- [Google Scholar]
- Many Hospitals Now Safely Reuse 'Single Use' Medical Devices. Available from: http://www.triplepundit.com/2010/03/reuse-single-use-medical-devices [Last accessed on 2022 Nov 29]
- [Google Scholar]
- Guidance on reuse of cardio-vascular catheters and devices in India: A consensus document. Indian Heart J. 2017;69:357-63.
- [CrossRef] [PubMed] [Google Scholar]
- Reuse of balloon catheters for coronary angioplasty: A potential cost-saving strategy? J Am Coll Cardiol. 1994;24:1475-81.
- [CrossRef] [PubMed] [Google Scholar]
- Infection risk of cardiac catheterization and arterial angiography with single and multiple use disposable catheters. Clin Cardiol. 1988;11:785-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cost-efficacy modelling of catheter reuse for percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1996;28:106-11.
- [CrossRef] [PubMed] [Google Scholar]
- The reuse of (single-use) cardiac disposable of coronary angiography and angioplasty: Safety and economic issues in Gaza strip. Online J Cardiol Res Rep. 2019;1
- [CrossRef] [Google Scholar]
- Report of the committee appointed by the Cardiological Society of India. Indian Heart J. 1997;49:329-31.
- [Google Scholar]
- Report to Congressional Requesters. In: Single-Use Medical Devices: Little Available Evidence of Harm from Reuse, but Oversight Warranted. Washington, D.C.: United States General Accounting Office; 2000. Available from: https://www.gao.gov/new.items/he00123.pdf [Last accessed on 2022 Nov 29]
- [Google Scholar]
- Safety and Performance Evaluation of Remanufactured Harmonic. Available from: http://www.amdr.org/wp-content/uploads/2010/09/white-paper-safety-and-performance-eval-of-remfd-harmonic-scalpels.pdf [Last accessed on 2022 Nov 29]
- [Google Scholar]
- Executive Summary Survey on the Reuse and Reprocessing of Single-Use Devices (SUDs) in US Hospitals. Available from: https://www.fda.gov/medicaldevices/deviceregulationandguidancereprocessingofsingle-usedevices/ucm121678.html [Last accessed on 2022 Nov 29]
- [Google Scholar]
- U.S. Government Accountability Office GAO-8-147 Reprocessed Single-Use Medical Devices. FDA Oversight Has Increased and Available Information Does Not Indicate that Use Presents an Elevated Health Risk. Available from: https://www.gao.gov/new.items/d08147.pdf [Last accessed on 2022 Nov 29]
- [Google Scholar]
- Initial experience with the reuse of coronary angioplasty catheters in the United States. J Am Coll Cardiol. 1997;30:1735-40.
- [CrossRef] [PubMed] [Google Scholar]
- Reuse of angiography catheters, Results and discussion of the present state of knowledge. Radiology. 1987;27:293-6.
- [Google Scholar]
- Clinical and angiographic procedural and mid-term outcome with new versus reused balloon catheters in percutaneous coronary interventions. Indian Heart J. 2005;57:114-20.
- [Google Scholar]
- One-stage coronary angiography and angioplasty. Am J Cardiol. 1995;75:30-3.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized study of the safety and efficacy of reused angioplasty balloon catheters. Indian Heart J. 2001;53:167-71.
- [Google Scholar]
- Absence of increased in-hospital complications with reused balloon catheters. Am J Cardiol. 1996;78:717-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reuse of pacing catheters: A survey of safety and efficacy. Pacing Clin Electrophysiol. 1988;11:1279-80.
- [CrossRef] [PubMed] [Google Scholar]
- Repeated use of ablation catheters: A prospective study. J Am Coll Cardiol. 1993;22:1367-72.
- [CrossRef] [PubMed] [Google Scholar]
- Ethylene oxide on electrophysiology catheters following resterilization: Implications for catheter reuse. Am J Cardiol. 1997;80:1558-61.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of hydrogen peroxide plasma sterilization for repeated use of electrophysiology catheters. J Am Coll Cardiol. 1998;32:1384-8.
- [CrossRef] [PubMed] [Google Scholar]
- Success of re-use of cardiac electrode catheters. Am J Cardiol. 1987;60:807-10.
- [CrossRef] [PubMed] [Google Scholar]
- Trends and patterns in electrophysiologic and ablation catheter reuse in the United States. Am J Cardiol. 2001;87:351-3.A9.
- [CrossRef] [PubMed] [Google Scholar]







