Translate this page into:
Plasma Homocysteine Levels as Cardiovascular Disease Risk vis-a-vis Estrogen Levels in Pre and Postmenopausal Women
*Corresponding author: Likhitha Munnangi, Department of Biochemistry, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India. likhithamunnangi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Munnangi L, Sai Baba KS, Mohammed N, Sai Satish O, Vijaya Bhaskar M, et al. Plasma Homocysteine Levels as Cardiovascular Disease Risk vis-a-vis Estrogen Levels in Pre and Postmenopausal Women. Indian J Cardiovasc Dis Women. 2024;9:143-9. doi: 10.25259/IJCDW_72_2023
Abstract
Objectives:
The objective of the study is to evaluate plasma homocysteine level and its relation with serum estrogen in pre and postmenopausal women as a risk factor in coronary artery disease (CAD).
Materials and Methods:
A cross-sectional study was conducted among premenopausal (n = 50) and postmenopausal women (n = 50) with an estimation of plasma homocysteine (Hcy) and serum estradiol (E2). Postmenopausal women based on their angiographic status were sub-grouped into A (with normal coronaries) and B (with CAD).
Results:
The levels of Hcy, 21.98 (20.89–24.05) μmol/L, increased significantly (P < 0.0001) and that of E2, 3.05 (2.32–3.6) pg/mL, decreased significantly (P = 0.0001) in the postmenopausal group when compared to Hcy, 6.11 (4.94–8.27) μmol/L and E2, 26.37 (25.3–29) pg/mL in premenopausal group. The levels of Hcy were elevated (24.7 ± 3.92 μmol/L) in postmenopausal Group B in comparison with postmenopausal Group A (21.37 ± 3.31 μmol/L), P = 0.002. Logistic regression analysis showed Hcy and body mass index to be independent predictors of CAD. Plasma Hcy showed an area under curve (AUC) of 1.00 with sensitivity and specificity of 100% at cutoff >12.6 μmol/L between pre and postmenopausal groups and AUC of 0.722 with 60% sensitivity and 84% specificity at cutoff >23.7 μmol/L between postmenopausal Groups A and B.
Conclusion:
We observed low E2 and high Hcy levels in postmenopausal women in comparison with premenopausal women. Among postmenopausal women, those with CAD had higher Hcy levels. Assessing Hcy levels routinely in postmenopausal women will help in better risk prediction of CAD and may also help in conjunction with other risk factors to decide the initiation of hormone replacement therapy.
Keywords
Homocysteine
Estradiol
Coronary artery disease
Premenopause
Postmenopause
ABSTRACT IMAGE
INTRODUCTION
Cardiovascular diseases (CVDs) emerged as a primary cause of death in India, constituting one-fourth of all mortalities. Over 80% of CVD-related deaths are due to ischemic heart disease and stroke. As per the Global Burden of Disease study, the age-standardized CVD death rate in India is 272/100,000 population, exceeding the global average of 235/100,000 population.[1] Indians experience CVDs about 10 years earlier than the Western population. The specific concerns for Indians regarding CVD include early onset, rapid progression, and a high mortality rate.[2]
Coronary artery disease (CAD) is the leading cause of mortality and morbidity accounting for one-third of total deaths in men and women and it has reached epidemic proportions among Indians. Regardless of race or ethnicity, CAD accounts for one out of three deaths among women with a high annual mortality rate. INTERHEART, a worldwide large cohort study of 52000 individuals with myocardial infarction (MI), revealed early CAD occurrence (around 10 years earlier) in women commonly after attaining menopause compared to men. Moreover, women exhibit poorer prognosis and more severe outcomes than men following events such as MI, percutaneous coronary intervention, and coronary artery bypass grafting. After the first episode of MI, the mortality is higher in women than men, and among survivors, there is a higher risk of recurrent MI, heart failure, or death.[3]
Aging is a primary risk factor for CAD. In women during menopausal transition, alterations in gonadal hormones exacerbate vascular aging. It is crucial to comprehend the factors contributing to endothelial dysfunction during menopause and to develop effective interventions that can preserve vascular well-being and prevent CAD in women. Among various risk factors, homocysteine (Hcy) is significantly associated with endothelial dysfunction. Studies showed that estrogen deficiency may contribute to elevated Hcy levels as women age.[4]
Hcy, a sulfur-containing amino acid (Mol Wt: 135.18 g/mol), is produced as an intermediate of methionine metabolism. Hcy metabolism is well regulated with its physiological level ranging between 5 and 15 μmol/L. Hcy levels >15 μmol/L are considered as hyperhomocysteinemia. Hcy is metabolized in the body by remethylation to methionine, transsulfuration to cysteine, and these pathways are catalyzed by methionine synthase, methylenetetrahydrofolate reductase (MTHFR), and cystathionine-beta-synthase (CBS) enzymes.
Hcy is an unstable amino acid prone to spontaneous oxidation resulting in the formation of reactive oxygen species (ROS) such as hydrogen peroxide and superoxide radicals. ROS subsequently oxidizes low-density lipoprotein cholesterol (LDL-C) and proteins. ROS formation and antioxidant activity are well regulated. Hcy signifies oxidative stress, suggesting that hyperhomocysteinemia leads to increased generation of ROS. Hcy thiolactone, a highly reactive compound arises as an intermediate of Hcy oxidation and its formation remains low at physiological levels of Hcy but increases significantly in hyperhomocysteinemia. Hcy thiolactone undergoes protein acylation of lysine residues and subsequent oxidation of LDL. Independently of the caspase pathway, Hcy thiolactone triggers endothelial cell apoptosis.[5]
Hyperhomocysteinemia can contribute to the development of CAD through various mechanisms: damage to cells, endothelium, and DNA, decreased glutathione peroxidase (GPx) activity, increased smooth muscle proliferation, facilitation of endothelial-leukocyte interactions, procoagulant activity stimulation, interference with anticoagulant and fibrinolytic pathways.[6]
There is limited data on cardiovascular disease risk and comparative studies on Hcy levels in relation to estrogen among Indian premenopausal and postmenopausal women.
Aims and objectives
The aims and objectives of the study are to evaluate plasma Hcy and its relation with serum estrogen levels in premenopausal and postmenopausal women as a risk factor in CAD.
MATERIALS AND METHODS
A cross-sectional study was conducted among premenopausal and postmenopausal women aged 25–65 years, attending the Cardiology department, Nizam’s Institute of Medical Sciences, and from the general population after obtaining Institutional Ethics Committee approval.
After obtaining informed consent, 100 subjects (n = 100) were recruited for the study and grouped into premenopause (n = 50) and postmenopause (n = 50). Postmenopausal women were sub-grouped based on angiographic findings: Group A (n = 25) – angiographically normal coronaries and Group B (n = 25) – angiographically proven CAD. Subjects on hormone replacement therapy (HRT), with pregnancy, menstrual disorders, renal insufficiency, diabetes mellitus, hypothyroidism, gastrointestinal disorders, history of smoking, alcohol consumption and on multivitamin supplements for the past 3 months, were excluded from the study.
A detailed history was taken from participants, body mass index (BMI) was calculated, and fasting blood samples were collected from premenopausal women in mid follicular phase (day 7–day 10[4]) of the menstrual cycle and from postmenopausal women (with 1-year complete cessation of menstruation[7]). Plasma Hcy and serum estradiol (E2) were measured by chemiluminescence assay. Data related to lipid profile, liver function, renal function, and hemogram (to rule out macrocytic anemia) were collected to assess other risk factors of CAD from participants’ records.
Statistical analysis was performed using MedCalc Software and Microsoft Excel. Distribution of data was evaluated by Kolmogorov-Smirnov test. Parametric data are presented as mean ± standard deviation and non-parametric data as median and inter-quartile range. Difference between variables of groups was analyzed by Student’s t-test and Mann–Whitney U-test for parametric and non-parametric data, respectively. Spearman’s rank correlation was performed to assess the correlation between continuous variables. Logistic regression analysis was performed to estimate the strength of the relationship between dependent variables and independent variables and to predict the probability of cardiovascular risk. Receiver operating characteristic (ROC) curve analysis was performed to assess the cutoff value of Hcy in determining the risk of development of CAD. Probability (P) value < 0.05 is considered statistically significant.
RESULTS
Participants’ baseline characteristics are shown below. Statistically significant difference was observed for Age, BMI, Hcy, E2, triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and very low-density lipoprotein cholesterol (VLDL-C) between pre and postmenopausal groups [Table 1]. Among postmenopausal Groups A and B, a significant difference was observed for BMI and Hcy [Table 2]. Statistical difference between levels of E2 and Hcy among groups is shown in Figure 1.
| Variable | Premenopausal group (n=50) | Postmenopausal group (n=50) | P-value |
|---|---|---|---|
| Age (years) | 33.5 (30–36) | 58 (55.6–59) | < 0.0001 |
| BMI (kg/m2) | 23.94±2.21 | 25.76±2.36 | 0.0001 |
| Hcy (µmol/L) | 6.11 (4.94–8.27) | 21.98 (20.89–24.05) | < 0.0001 |
| E2 (pg/mL) | 26.37 (25.3–29) | 3.05 (2.32–3.6) | < 0.0001 |
| TG (mg/dL) | 106.5 (100.2–125.79) | 129 (109.81–156.77) | 0.02 |
| TC (mg/dL) | 168.92±35.29 | 190.52±38.46 | 0.004 |
| HDL-C (mg/dL) | 45.92±7.89 | 50.26±11.01 | 0.026 |
| VLDL-C (mg/dL) | 21 (20–25.39) | 26 (22–31.39) | 0.02 |
| LDL-C (mg/dL) | 99.24±31.09 | 110.56±33.65 | 0.08 |
BMI: Body mass index, E2: Estradiol, HDL-C: High-density lipoprotein cholesterol, Hcy: Homocysteine, LDL-C: Low-density lipoprotein cholesterol, TG: Triglyceride, TC: Total cholesterol, VLDL-C: Very low-density lipoprotein cholesterol
| Variable | Postmenopausal group A (n=25) | Postmenopausal group B (n=25) | P-value |
|---|---|---|---|
| Age (years) | 55 (52–59.85) | 58 (56–59) | 0.71 |
| BMI (kg/m2) | 24.64±2.15 | 26.87±2.05 | 0.0005 |
| Hcy (μmol/L) | 21.37±3.31 | 24.7±3.92 | 0.002 |
| E2 (pg/mL) | 2.93±1.49 | 2.91±1.48 | 0.97 |
| TG (mg/dL) | 121 (108.44–151.85) | 135 (98.14–219.86) | 0.86 |
| TC (mg/dL) | 185.88±37.46 | 195.16±39.65 | 0.39 |
| HDL-C (mg/dL) | 51±9.24 | 49.52±12.7 | 0.63 |
| VLDL-C (mg/dL) | 24 (22–30) | 27 (20–43.97) | 0.83 |
| LDL-C (mg/dL) | 107.44±36.69 | 113.68±30.75 | 0.51 |
BMI: Body mass index, E2: Estradiol, HDL-C: High-density lipoprotein cholesterol, Hcy: Homocysteine, LDL-C: Low-density lipoprotein cholesterol, TG: Triglyceride, TC: Total cholesterol, VLDL-C: Very low-density lipoprotein cholesterol

- Levels of estradiol (pg/mL) and homocysteine (μmol/L) in premenopausal and postmenopausal Groups A and B.
Hcy has demonstrated a strong negative correlation with E2 (r = –0.77, P < 0.0001), very strong positive correlation with age (r = 0.83, P < 0.0001), significantly weak positive correlation with BMI, TG, TC, and VLDL-C [Table 3].
| Variable | Correlation coefficient(r) | P-value |
|---|---|---|
| Age | 0.83 | <0.0001 |
| BMI | 0.34 | 0.0006 |
| E2 | −0.77 | < 0.0001 |
| TG | 0.28 | 0.0231 |
| TC | 0.23 | 0.0197 |
| HDL-C | 0.16 | 0.1022 |
| VLDL-C | 0.23 | 0.0234 |
| LDL-C | 0.14 | 0.1538 |
Hcy: Homocysteine, BMI: Body mass index, E2: Estradiol, HDL-C: High-density lipoprotein cholesterol, Hcy: Homocysteine, LDL-C: Low-density lipoprotein cholesterol, TG: Triglyceride, TC: Total cholesterol, VLDL-C: Very low-density lipoprotein cholesterol
E2 has shown a very strong negative correlation with age (r = −0.86, P < 0.0001) and significant weak negative correlation with BMI, TG, TC, and VLDL-C [Table 4].
| Variable | Correlation coefficient(r) | P-value |
|---|---|---|
| Age | −0.86 | <0.0001 |
| BMI | −0.36 | 0.0003 |
| Hcy | −0.77 | < 0.0001 |
| TG | −0.25 | 0.0129 |
| TC | −0.22 | 0.0258 |
| HDL-C | −0.12 | 0.2277 |
| VLDL-C | −0.25 | 0.0114 |
| LDL-C | −0.14 | 0.1606 |
BMI: Body mass index, E2: Estradiol, HDL-C: High-density lipoprotein cholesterol, Hcy: Homocysteine, LDL-C: Low-density lipoprotein cholesterol, TG: Triglyceride, TC: Total cholesterol, VLDL-C: Very low-density lipoprotein cholesterol
When logistic regression analysis was performed, Hcy and BMI were found to be independent predictors of CAD as shown [Table 5].
| Variable | OR | 95%CI | P-value |
|---|---|---|---|
| Age | 1.1042 | 0.9015–1.3525 | 0.3382 |
| BMI | 1.7451 | 1.1041–2.7582 | 0.0171 |
| Hcy | 1.3717 | 1.0952–1.7181 | 0.0059 |
| E2 | 0.9049 | 0.5581–1.4674 | 0.6855 |
| TC | 1.9545 | 0.6785–5.6299 | 0.2144 |
| TG | 0.8707 | 0.7035–1.0776 | 0.2030 |
| HDL-C | 0.4978 | 0.1720–1.4406 | 0.1982 |
| LDL-C | 0.5139 | 0.1777–1.4862 | 0.2192 |
BMI: Body mass index, CI: Confidence interval, E2: Estradiol, HDL-C: High-density lipoprotein cholesterol, Hcy: Homocysteine, LDL-C: Low-density lipoprotein cholesterol, OR: Odds ratio, TG: Triglyceride, TC: Total cholesterol, VLDL-C: Very low-density lipoprotein cholesterol
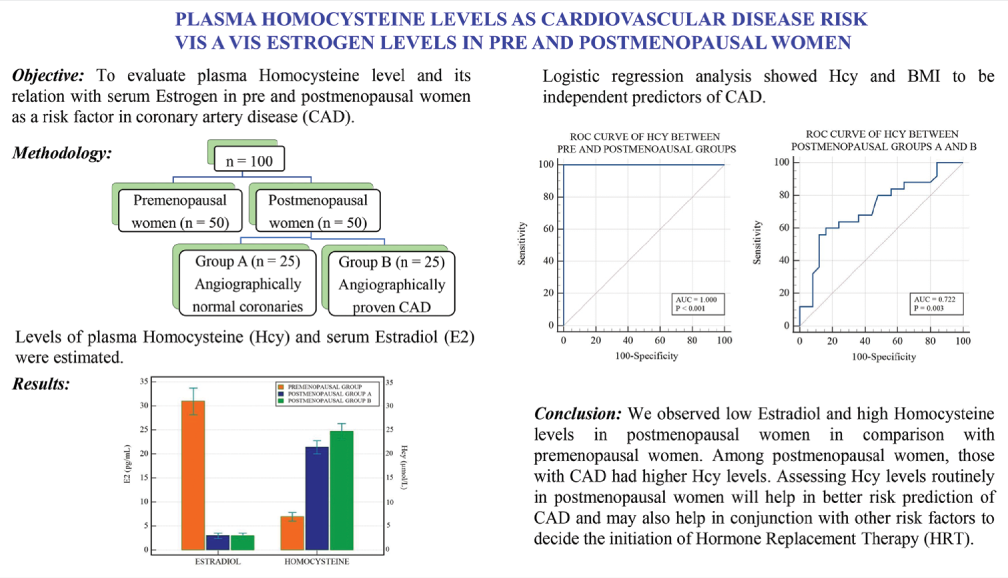
ROC curve analysis of Hcy between pre and postmenopausal groups showed 100% sensitivity and specificity with area under curve (AUC) of 1.0 at cutoff value >12.6 μmol/L [Figure 2]. ROC curve analysis of Hcy between postmenopausal Groups A and B showed 60% sensitivity and 84% specificity with an AUC of 0.722 at cutoff value >23.7 μmol/L [Figure 3]. A comparison of both ROC curves is shown in Table 6.
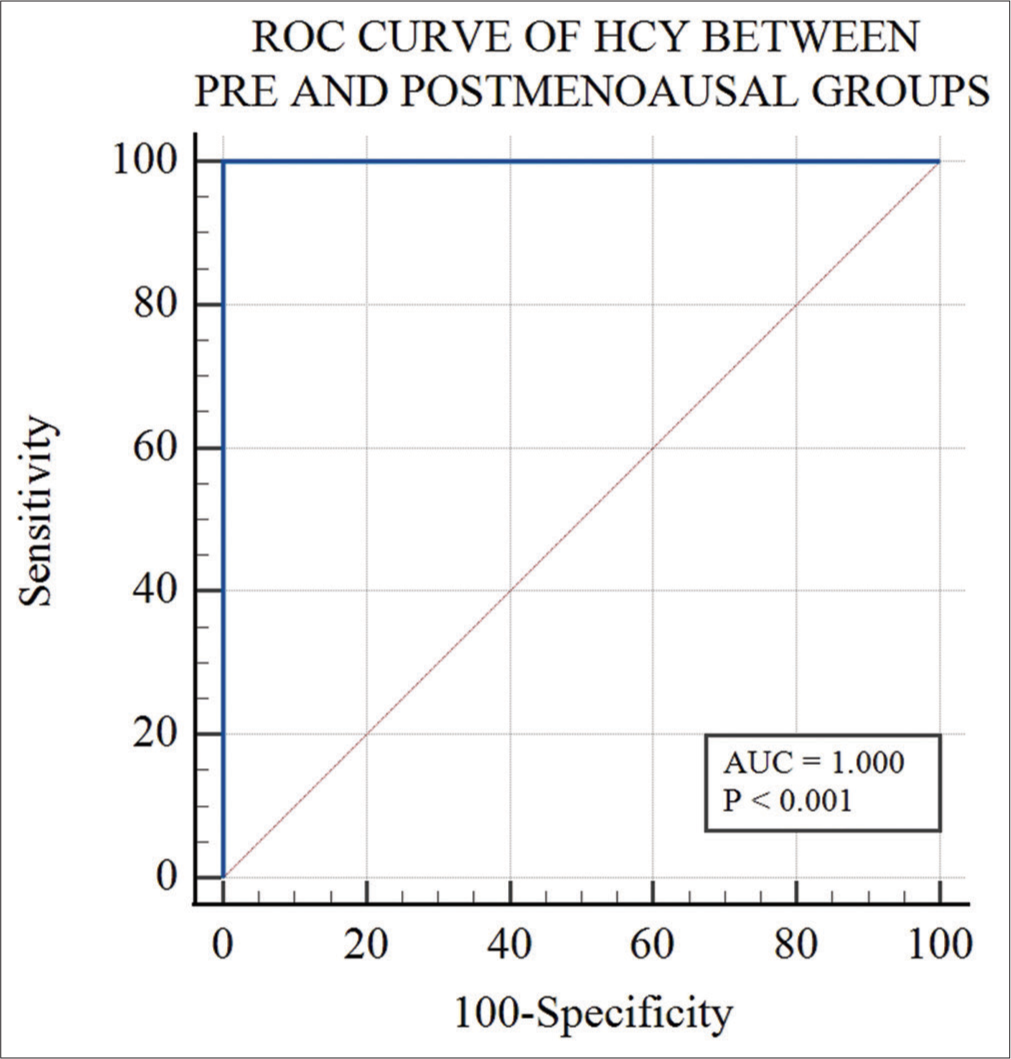
- Receiver operating characteristic (ROC) curve analysis of homocysteine between pre and postmenopausal groups. (AUC: Area under curve).
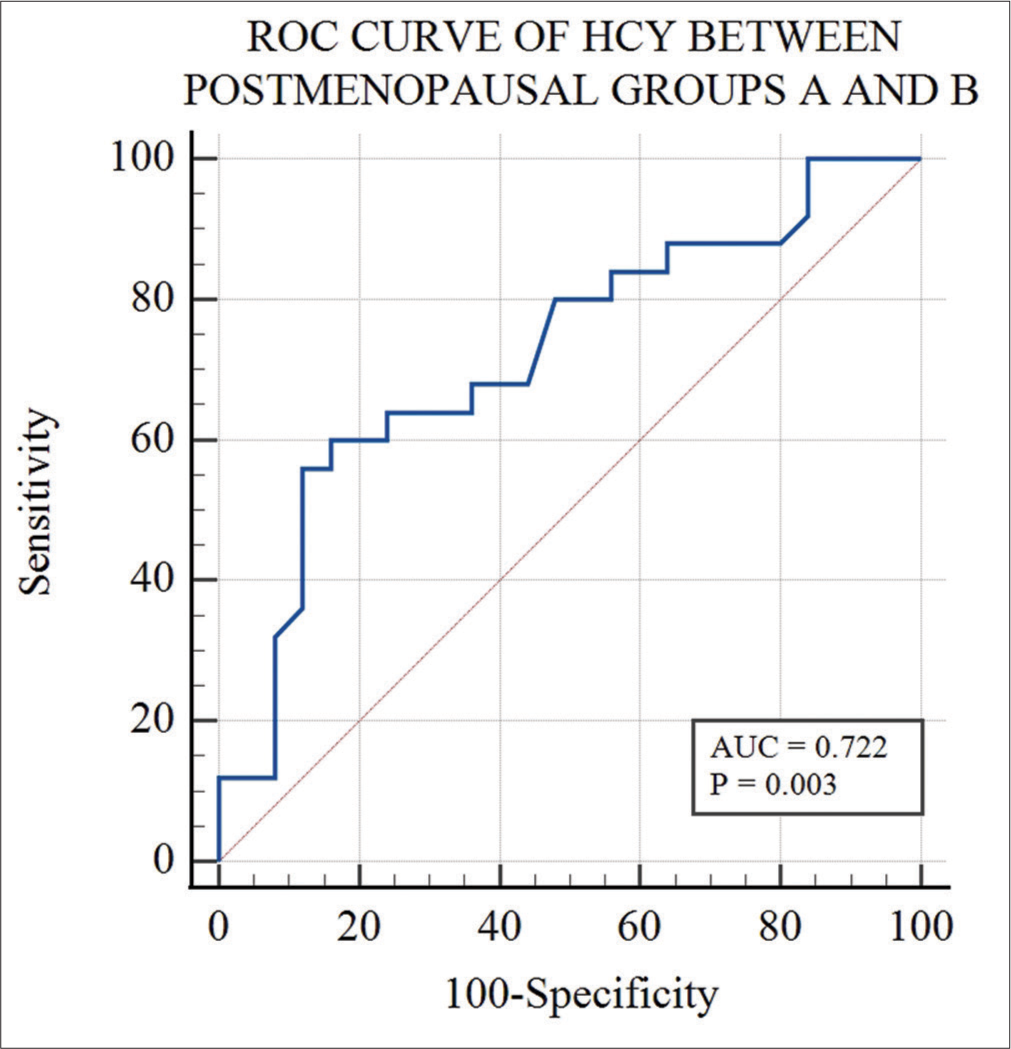
- Receiver operating characteristic (ROC) curve analysis of homocysteine between postmenopausal Groups A and B. (AUC: Area under curve)
| Group | AUC | Cutoff (μmol/L) | Sensitivity | Specificity |
|---|---|---|---|---|
| Premenopausal versus Postmenopausal groups | 1.000 | >12.6 | 100.00 | 100.00 |
| postmenopausal Group A versus Group B | 0.722 | >23.7 | 60.00 | 84.00 |
ROC: Receiver operating characteristic, AUC: Area under curve
DISCUSSION
In this study, we compared Hcy and E2 levels in premenopausal and postmenopausal women, postmenopausal women with CAD and without CAD. Results showed significantly decreased E2 levels and increased Hcy levels in postmenopausal women compared to premenopausal women. Postmenopausal Group B had higher Hcy levels compared to Group A. Postmenopausal women with such a combination of low estrogen and high Hcy are prone for development of CAD.
Elevated levels of Hcy pose an independent risk for CAD. In women, the aging process is linked to raised Hcy levels and research indicated that decreasing estrogen levels contribute to Hcy elevation. This suggests higher Hcy levels in postmenopausal women in comparison to premenopausal women.[4,8] Hak et al., stated that “plasma Hcy levels increase with natural menopause, which strengthens the hypothesis that estrogen influences Hcy levels and its adverse effects in the development of cardiovascular disease.” However, existing data showed inconsistencies, and studies published to date have not adequately adjusted for age, a crucial confounding variable while studying the impact of menopause.[9]
Some studies demonstrated lowered Hcy levels in postmenopausal women with hormonal therapy.
A significant reduction in Hcy was seen with combined hormonal therapy in postmenopausal women with higher baseline concentrations of Hcy.[10,11] Prolonged hormonal therapy in postmenopausal women with MTHFR genotypes resulted in lowered Hcy levels.[12] Hormonal therapy may exhibit a positive effect on cardiovascular disease risk reduction by significantly lowering Hcy levels. Effectiveness of hormonal therapy may depend on the age of the women and the period of its initiation after menopause.[13]
Estrogen up-regulates nitric oxide synthase (NOS) activity and increases nitric oxide (NO) production. Peroxynitrite (ONOO−), a highly cytotoxic molecule is generated in the presence of high NO concentrations and an abundance of superoxide. The protective action of estrogen is attributed to its dosage-dependent stimulation, resulting in elevated levels of myocardial and plasma glutathione (GSH). Elevated GSH levels prevent ONOO− formation by stabilizing NO in GSNO (s-nitrosoglutathione). GSNO, a redox form of NO, exerts cytoprotective effects. Estrogen increases GPx activity, which scavenges oxygen free radicals by conversion of GSH (reduced) to GSSG (oxidized). Estrogen additionally increases glucose 6-phosphate dehydrogenase activity, resulting in nicotinamide adenine dinucleotide phosphate (NADPH) generation. NADPH is a co-factor for NOS and helps replenish GSH availability (reduces GSSG to GSH). Moreover, estrogen enhances CBS activity, redirecting Hcy metabolism toward the formation of cysteine and GSH. Consequently, by modulating the transsulfuration pathway, estrogen reduces Hcy levels.[8] These above mechanisms explain the influence of estrogen on Hcy and its role in preventing the development of CAD. As women age, the decline in estrogen levels may contribute to increased Hcy levels. This supports the results of our study.
The other causes affecting Hcy metabolism leading to hyperhomocysteinemia are genetic factors, nutritional deficiencies, and chronic diseases. Genetic factors include MTHFR, and CBS defects. CBS defects can be treated with vitamin B6 along with folate and betaine. Nutritional states include vitamin B12, B6, and folate deficiencies.[14] It is difficult to maintain a folate requirement of 0.4 mg/day even with an adequate balanced diet.[15] To attain the maximum decrease in plasma Hcy levels, a daily dosage of 0.8 mg of folate supplementation is necessary.[16] Randomized clinical trials with oral supplementation of vitamins–Folate, B6, and B12 have shown significant reduction in Hcy levels.[17] Hcy levels should be estimated and treated at an early age as folate is easily available and has the ability to reduce the risk of vascular diseases.[18] To account for the factors influencing Hcy metabolism, we excluded nutritional deficiencies and chronic diseases.
Hyperhomocysteinemia causes an increase in the synthesis and release of TG and cholesterol, but inhibits the synthesis of apolipoprotein A1, a primary apolipoprotein of HDL-C, leading to reduced levels of HDL-C.[19] The observed elevated levels of TG and TC, along with decreased HDL-C in postmenopausal Group B compared to Group A in our study, may be attributed to these mechanisms.
Limitations
Non-estrogen-related causes of hyperhomocysteinemia like genetic enzyme defects in the Hcy metabolic pathways could not be excluded from the study
This is a single-center study; hence, we need more such studies in different regions to generalize the conclusions.
CONCLUSION
In our study, we observed high plasma Hcy and low E2 in the postmenopausal group. Plasma Hcy assessment in women enables more reliable risk stratification. Plasma Hcy has a greater clinical significance in the causation of CAD and its complications. Assessment of plasma Hcy levels may help in decision-making to initiate HRT after thorough workup among postmenopausal women. Efficient recognition and management of risk factors in CAD are important and will be cost-effective. The role of HRT in the reduction of cardiovascular disease risk among postmenopausal women with high Hcy levels needs to be evaluated in further studies.
Ethical approval
The research/study was approved by the Institutional Review Board at Nizam’s Institute of Medical Sciences, number EC/NIMS/3349/2023, dated 13/12/2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Audio summary available at
Financial support and sponsorship
Nil.
References
- Cardiovascular Diseases in India. Circulation. 2016;133:1605-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular Disease in India: A 360 Degree Overview. Med J Armed Forces India. 2020;76:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary Artery Disease in Women. Indian Heart J. 2017;69:532-8.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated Plasma Homocysteine and Cysteine are Associated with Endothelial Dysfunction across Menopausal Stages in Healthy Women. J Appl Physiol (1985). 2019;126:1533-40.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Homocysteine and Vascular Calcification: Advances in Mechanisms, Related Diseases, and Nutrition. Korean J Fam Med. 2022;43:277-89.
- [CrossRef] [PubMed] [Google Scholar]
- Homocysteine and Atherothrombosis-Mechanisms for Injury. Eur Heart J. 2000;21:967-74.
- [CrossRef] [PubMed] [Google Scholar]
- Age of Menopause and Determinants of Menopause Age: A PAN India Survey by IMS. J Midlife Health. 2016;7:126-31.
- [CrossRef] [PubMed] [Google Scholar]
- Increased Plasma Homocysteine after Menopause. Atherosclerosis. 2000;149:163-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hormone Replacement Therapy may Reduce High Serum Homocysteine in Postmenopausal Women. Eur J Clin Invest. 1994;24:733-6.
- [CrossRef] [PubMed] [Google Scholar]
- Postmenopausal Oral 17beta-Estradiol Continuously Combined with Dydrogesterone Reduces Fasting Serum Homocysteine Levels. Fertil Steril. 1998;69:876-82.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Long-term Hormone Replacement Therapy on Plasma Homocysteine in Postmenopausal Women: A Randomized Controlled Study. Am J Obst Gynecol. 2002;187:33-9.
- [CrossRef] [PubMed] [Google Scholar]
- What is the Influence of Hormone Therapy on Homocysteine and CRP Levels in Postmenopausal Women? Clinics (Sao Paulo). 2015;70:107-13.
- [CrossRef] [PubMed] [Google Scholar]
- Homocysteine-Lowering Treatment: An Overview. Expert Opin Pharmacother. 2001;2:1449-60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Use and Rational Management of Homocysteine, Folic Acid, and B Vitamins in Cardiovascular and Thrombotic Diseases. Z Kardiol. 2004;93:439-53.
- [CrossRef] [PubMed] [Google Scholar]
- Dose-Dependent Effects of Folic Acid on Blood Concentrations of Homocysteine: A Meta-analysis of the Randomized Trials. Am J Clin Nutr. 2005;82:806-12.
- [CrossRef] [PubMed] [Google Scholar]
- The Treatment of Hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
- [CrossRef] [PubMed] [Google Scholar]
- Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv Ther. 2020;37:4149-64.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma Homocysteine Level Is Independently Associated With Conventional Atherogenic Lipid Profile and Remnant Cholesterol in Adults. Front Cardiovasc Med. 2022;9:898305.
- [CrossRef] [PubMed] [Google Scholar]








