Translate this page into:
Right Coronary Artery Aneurysm Masquerading as a Pericardial Cyst: A Case Report
*Corresponding author: Praveen Nagula, Associate Professor, Department of Cardiology, Osmania Medical College/General Hospital, Hyderabad, Telangana, India. drpraveennagula@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bharath K, Parvathareddy MRK, Nagula P, Avinash B. Right Coronary Artery Aneurysm Masquerading as a Pericardial Cyst: A Case Report. Indian J Cardiovasc Dis Women. 2024;9:112-5. doi: 10.25259/IJCDW_11_2024
Abstract
Coronary artery aneurysms (CAAs) or ectasias are dilatations of arterial segments, commonly detected as an incidental finding during angiography. The incidence of CAAs varies from 0.3% to 5.3%, mainly caused by atherosclerosis, though infections, inflammatory conditions, and connective tissue disorders can also contribute. Giant CAAs are rare with a reported prevalence of 0.02–0.2%. In our case, a 45-year-old female has been diagnosed with a giant aneurysm in the right coronary artery, which was initially misinterpreted as a pericardial cyst. In addition, she has a bicuspid aortic valve and severe aortic stenosis, hence advised for surgical management.
Keywords
Giant coronary artery aneurysms
Coronary artery aneurysm
Right coronary artery
Pericardial cyst
INTRODUCTION
Coronary artery aneurysms (CAAs), characterized by localized arterial dilatation, have seen increased incidence in the interventional era, primarily linked to diverse causes such as atherosclerosis, prior myocardial infarction, and interventions such as drug-eluting stent placement.[1] The range of etiologies also includes connective tissue disorders, vasculitis, certain infections, drugs, Kawasaki disease in childhood, and congenital aneurysms.[2]
In those with CAA, the most frequent aneurysmal finding is right coronary artery (RCA) dilation followed by the left anterior descending (LAD) artery dilation. Notably, the diagnosis of giant aneurysms may be challenging, occasionally misinterpreted as mediastinal masses or cysts, potentially delaying crucial treatment. Such delays can lead to severe complications such as rupture, fistula formation, or compression of cardiac chambers, emphasizing the need for accurate and timely diagnosis.
CAAs indeed pose challenges in their management due to the lack of large randomized clinical trials. Relying on case reports and smaller studies underscores the need for a personalized treatment approach.
CASE REPORT
A 45-year-old female presented with a gradual onset of worsening chest pain and shortness of breath over 10 months. After being referred to a cardiologist, she was diagnosed with severe aortic stenosis due to a bicuspid aortic valve and was also found to have a large pericardial cyst. Seeking further treatment, she was admitted to our hospital.
On general examination, an ejection click and a harsh systolic murmur in the left second intercostal space were present. Her biochemical parameters were within normal range. Electrocardiogram showed sinus rhythm, left axis deviation, and signs of left ventricular hypertrophy with strain. Echocardiography confirmed the left ventricular hypertrophy and identified bicuspid aortic valve and severe aortic stenosis.
A hypoechoic lesion near the right atrium with pulsatile flow was observed on pulse wave Doppler during the echocardiogram [Figures 1 and 2]. However, color Doppler imaging did not display any mosaic pattern. A cardiac computed tomography (CT) scan was performed, which revealed a cystic lesion of 25 cm2 area adjacent to the right atrium [Figure 3]. The differential diagnosis included a pericardial cyst, true aneurysm, or pseudoaneurysm of the RCA. Subsequent CT coronary angiography disclosed a giant coronary artery saccular aneurysm measuring 6 cm in transverse diameter, originating from the RCA [Figure 3].
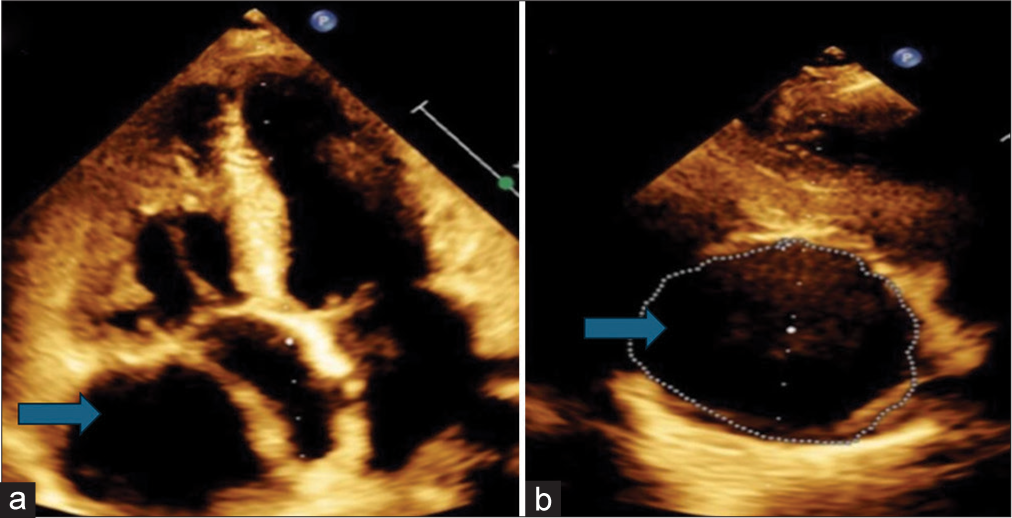
- (a and b) Hypoechoic mass measuring 4.93 cm (as shown by blue arrow) in maximum length and 1(b) having an area of 25.2 cm2 as shown by blue arrow.
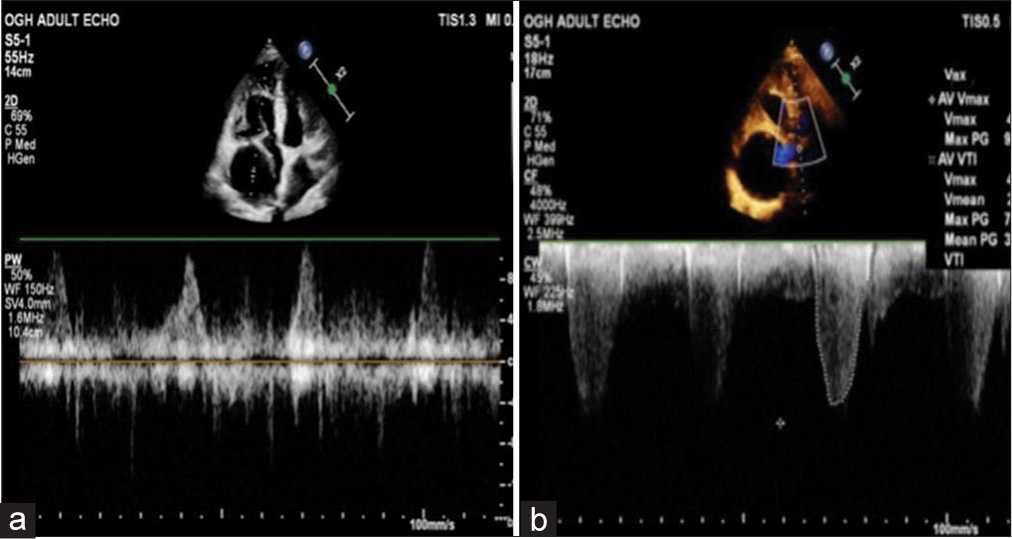
- (a) Pulse wave Doppler within the hypoechoic mass showing pulsatile flow pattern and (b) continuous wave Doppler (CWD) at the level of the aortic valve showing severe aortic stenosis.

- (a) Three-dimensional (3D) reconstruction of cardiac computed tomography showing a large aneurysm arising from the right coronary artery (RCA) as shown by blue arrow. (b) Whole body 3D reconstruction confirming the origin of aneurysm from RCA) as shown by blue arrow.
The proposed treatment plan involved aortic valve replacement and excision of the aneurysm while ensuring continuity of the RCA using a saphenous venous interposition graft.
DISCUSSION
CAAs are defined as localized expansions of an artery, surpassing 1.5 times the diameter of the neighboring normal vessel or exhibiting a 50% increase in diameter compared to the adjacent arterial segment.[3] The incidence of CAAs varies from 0.3% to 5.3%.[4] The causes of CAAs encompass various factors such as atherosclerosis, trauma, congenital malformations, infections, and vasculitic diseases.[1,2]
CAAs are classified according to their size and their extent of involvement. The classification of CAA is described in Table 1 and Figure 4.[5,6] Giant CAAs, characterized by vessel dilation measuring 2 cm or more, are extremely rare, occurring at a reported rate of 0.02% in a cardiac surgical population, with a higher prevalence in the RCAs than the left.[7] Simultaneous RCA and LAD aneurysms are extremely rare, and often linked to Kawasaki disease. Giant coronary aneurysms are more likely to be congenital as opposed to smaller CAAs in which the significant majority are atherosclerotic or vasculitic (e.g., Kawasaki’s disease) by origin. Their clinical presentation can vary widely, from being asymptomatic to resembling ischemic heart disease. Failure to promptly recognize or manage giant CAA can lead to complications such as vessel thrombosis causing myocardial ischemia or aneurysmal rupture resulting in acute pericardial tamponade.[7] Differential diagnoses for giant coronary aneurysm include aneurysm of the heart wall, post-traumatic pseudoaneurysm affecting the ascending aorta or pulmonary trunk, cardiac or pericardial tumors, and thymoma.
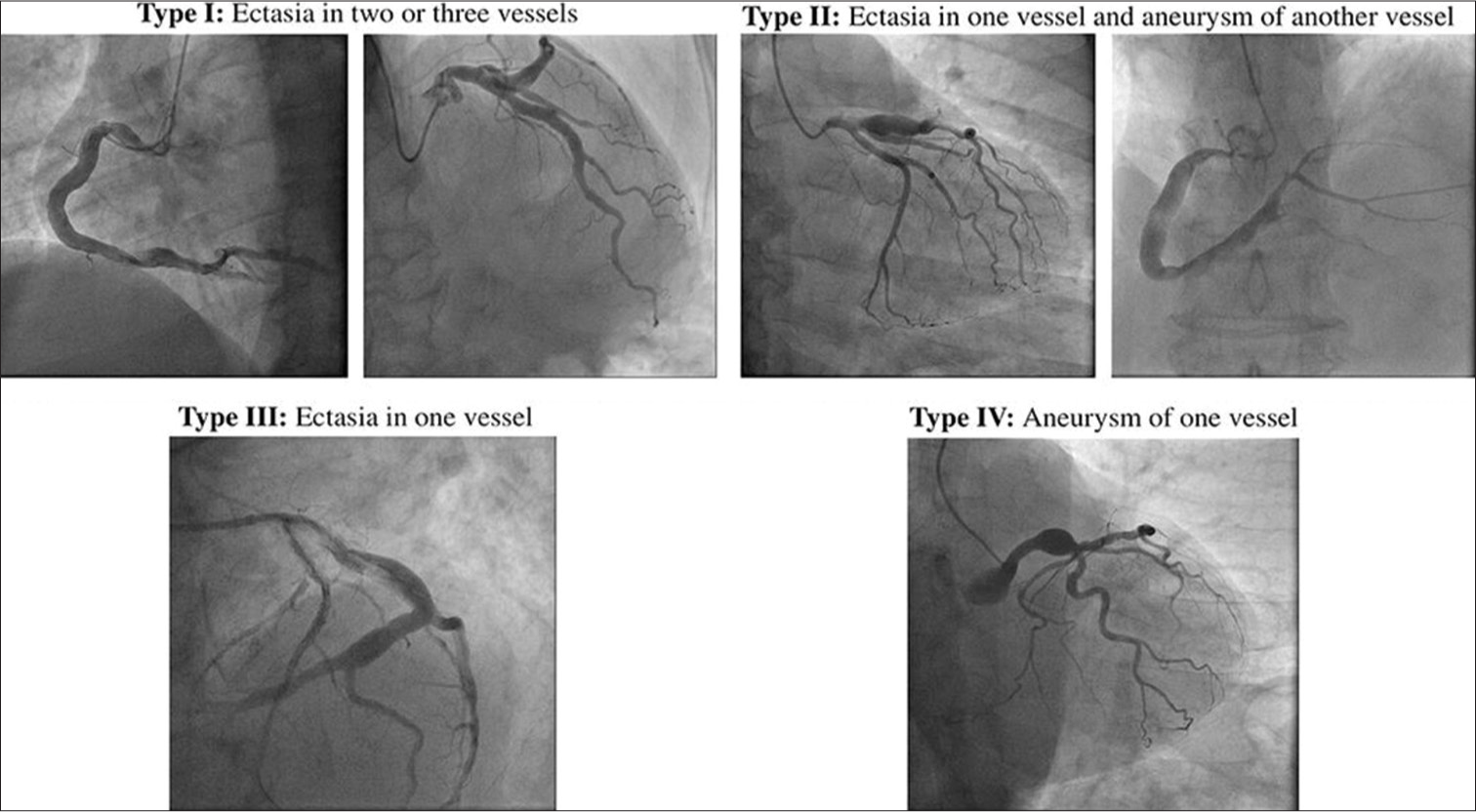
- Markis classification of coronary artery aneurysm.
| Based on the size | |
| Giant aneurysm (adults) | >20–150 mm in diameter |
| Giant aneurysm (children) | >8 mm in diameter |
| Based on the extent of involvement (Markis classification)[6] | |
| Type I | Diffuse dilatation of two or three vessels |
| Type II | Diffuse dilatation in one vessel and localized disease in an another |
| Type III | Diffuse dilatation of one vessel only |
| Type IV | Localized or segmental dilatation |
Coronary angiography is commonly used for assessing ecstatic or aneurysmal coronary arteries, but challenges like delayed contrast filling can hinder accurate imaging. Intravascular ultrasound (IVUS) provides better vessel wall visualization and aids in distinguishing between true and pseudoaneurysms. IVUS also helps in sizing CAAs and adjacent stenoses for potential percutaneous coronary intervention. Caution is needed with post-stenting IVUS to avoid stent dislodgement. Optical coherence tomography’s use in CAA assessment is limited due to its small scan diameter. Coronary CT is increasingly preferred for its precise evaluation of aneurysm size, thrombus, and calcification, particularly beneficial for giant CAAs and coronary artery fistulas.[8]
Conventionally, anticoagulation has been the primary treatment for aneurysms measuring <20 mm, while surgical intervention remains the key approach for managing giant CAAs.[9] Surgical intervention for CAAs is often imperative, particularly in cases where alternative treatments are inadequate. These instances include severe coronary artery disease coupled with valve disease, CAAs positioned proximal to major branch bifurcations, and those located within the left main stem. In addition, multiple or markedly enlarged CAAs, mechanical complications such as fistula formation or compression of neighboring cardiac or vascular structures, and a high risk of rupture further necessitate surgical management. CAAs arising from Kawasaki disease, particularly those with specific characteristics such as ostial, multiple, or long-segment stenosis, or those complicated by infection, also warrant surgical consideration. Furthermore, aortocoronary saphenous vein graft aneurysms showing symptoms or significant enlargement, as well as diminished graft flow, often require surgical intervention to mitigate potential complications and ensure optimal patient outcomes.
Although the previous literature has reported cases of CAAs, this specific case is remarkable due to its initial misdiagnosis as a pericardial cyst and its extraordinary size. The exact incidence of CAAs falsely presenting as a pericardial cyst is unknown, but there are very few case reports of CAAs presenting as a mediastinal mass or pericardial cyst in the literature.[10] In addition, its association with severe aortic stenosis adds complexity to its clinical presentation, making it a rare and intriguing occurrence.
CONCLUSION
CAAs can arise from diverse causes. Swift attention is imperative for addressing symptomatic giant aneurysms to avert potential catastrophic consequences.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- A Case of Giant Coronary Artery Aneurysm and Literature Review. J Cardiol. 2009;53:293-300.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 2018;11:1211-23.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary Artery Aneurysms and Ectasia: Role of Coronary CT Angiography. Radiographics. 2009;29:1939-54.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Significance of Coronary Arterial Ectasia. Am J Cardiol. 1976;37:217-22.
- [CrossRef] [PubMed] [Google Scholar]
- MDCT of Coronary Artery Aneurysms. Am J Roentgenol. 2005;184:S19-20.
- [CrossRef] [PubMed] [Google Scholar]
- Management of a Single Coronary Artery Aneurysm by Use of a Stent. Proc (Bayl Univ Med Cent). 2002;15:255-6.
- [CrossRef] [PubMed] [Google Scholar]
- Giant Coronary Artery Aneurysm Presenting as a Mediastinal Mass. Am J Cardiol. 1998;82:1307-8.
- [CrossRef] [PubMed] [Google Scholar]








