Translate this page into:
Long-Term Cardiovascular Effects of Pregnancy-Related Disorders
Jyotsna Maddury, MD, DM Nizam’s Institute of Medical Sciences Punjagutta, 500082, Hyderabad India mail2jyotsna@rediffmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Usually, pregnancy-elated effects and changes in the different organs terminate after delivery or maybe within 6 months. Long-term effect of complicated pregnancies leading to long-term cardiovascular and other diseases was recognized long back. With the accumulating evidence with many landmark studies, it became mandatory to have pregnancy heart team approach not only to manage during pregnancy and peripartum period but also to monitor future events and educate the pregnant women about the modification of cardiovascular risks for prevention of anticipated events. In this review, more importance is given to risk-stratify these women with complicated pregnancy and recommendation to prevent the long-term effects.
Keywords
cardiovascular effects
gestational diabetes
preeclampsia
pregnancy
Introduction
The changes that occur during pregnancy are expected to terminate their effect after delivery, which sometimes may extend up to 6 weeks after delivery, for example preeclampsia, gestational diabetics, etc. Much was discussed on recurrence of some diseases such as preterm labor or preeclampsia that have greater chances of recurrence in subsequent pregnancies. However, in the recent past, it was realized that the events that occurred during pregnancy predicted the development of future events in the life.1
Similarly, it is well known that obstetric complications are related to long-term complications of the newborn. However, Pariente et al and Almasi et al showed that the small for gestational baby birth was the predictor for maternal cardiovascular disease (CVD) later in the life.2 3 In the mother, the cause of the long-term effects of the pregnancy was debated by many researchers. Some authors suggest that the longterm effects may not be due to the pregnancy as such, but it may be due to the expression of already existing predisposing factors during pregnancy. In addition, some authors say that pregnancy acts as a long term stress (9 months) that may have effects on the long-term maternal health.4
In this review article, the authors discussed the different pregnancy-related problems with their pathophysiology, which may be responsible for long-term effect on the cardiovascular system of the mother with evidence of previous studies.
Gestational Diabetes
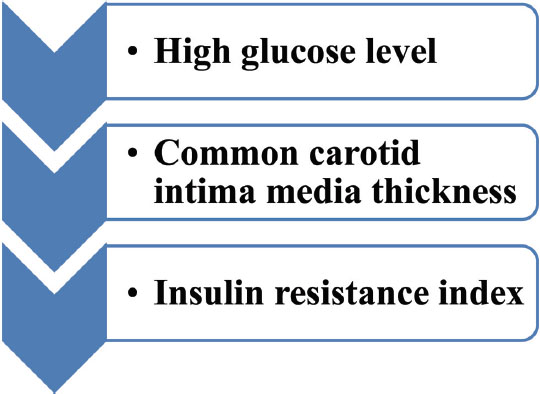
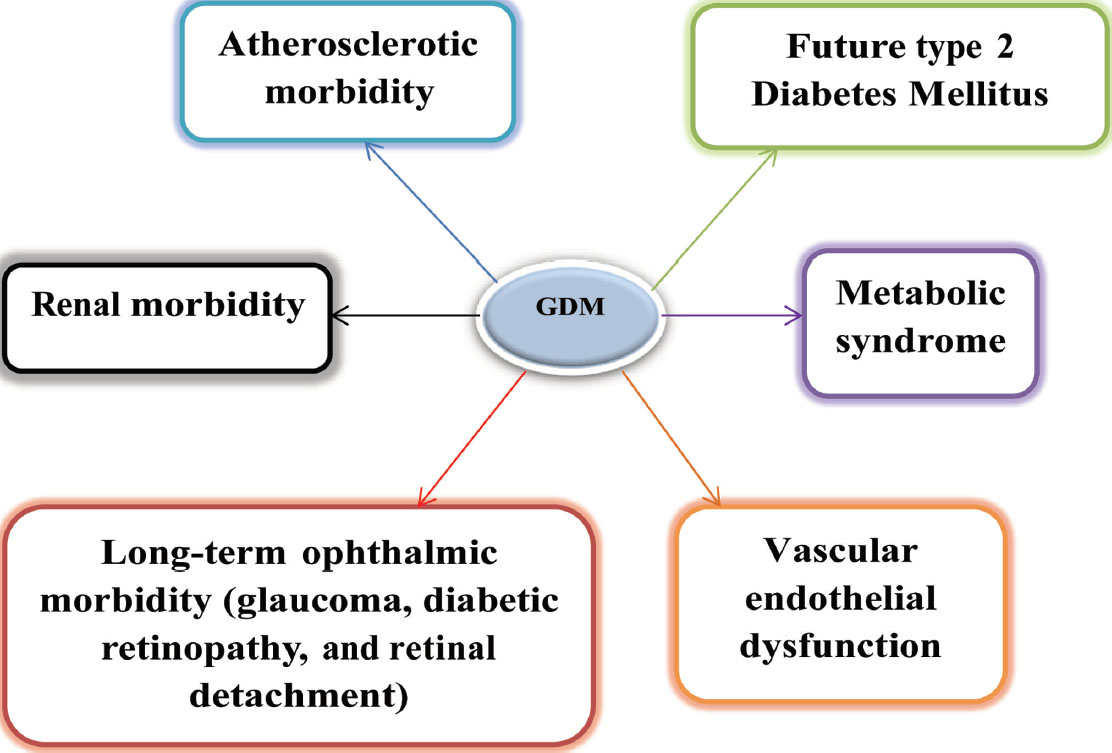
Glucose tolerance test was performed on the patients who had the previous history of gestational diabetes by giving 75 g of glucose 6 to 12 weeks after delivery. Approximately 2 to 16% patients were detected to be type 2 diabetic, and 36% of patients had an intolerance to carbohydrates. Therefore, nearly 36 to 70% of GDM (gestational diabetes mellitus) women were prone to type 2 diabetes in the long run1 (Fig. 1) and GDM itself can be precursor of future events (Fig. 2).
-
Fig. 1 Markers in GDM patients to detect the CVD subsequently.
Fig. 1 Markers in GDM patients to detect the CVD subsequently.
-
Fig. 2 GDM—future events.
Fig. 2 GDM—future events.
Studies that concentrated on the subsequent development of type 2 diabetes mellitus (DM) and another cardiovascular morbidity in GDM women are mentioned in Table 1.5 6 7 8 9 10 11
|
Study author |
Year |
Inclusion no. |
Results |
|---|---|---|---|
|
Abbreviations: BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; CHD, coronary heart disease; DM, diabetes mellitus; GD, gestational diabetes; GDM, gestational diabetes mellitus; HbA1C, hemoglobin A1C; HDL, high–density lipoprotein; OR, odds ratio. |
|||
|
Lauenborg et al |
2005 |
9.8-y follow–up—481 Danish women; prior diet–treated GDM after pregnancy |
Metabolic syndrome: 1. Prior diet–treated GDM 3-fold > age-matched control patients (p < 0.0005). 2. Obese women (BMI > 30) with previous GDM 7-fold > normal-weight prior GDM women (BMI< 25) 3. Prevalence—glucose tolerant GDM group > 2 times control group |
|
Bellamy et al |
2009 |
Comprehensive systematic review and meta-analysis |
Gestational diabetes ↔ risk of type 2 diabetes |
|
Göbl et al |
2011 |
10-y study |
Glucose tolerance—impaired in GD predictors of DM after GDM, HDL cholesterol < 50 mg/dL and age (> 35 y) |
|
Lee et al |
2012 |
15 prospective studies—760,925 participants |
Prediabetes—↑stroke—impairment of glucose tolerance/combination of impaired fasting glucose and glucose tolerance |
|
Kessous et al |
2013 |
47,909 10-y follow–up |
4,928 (10.3%) GDM—↑rate of CV morbidity—noninvasive cardiac diagnostic procedures (OR = 1.8; 95% CI: 1.4–2.2), simple CV events (OR = 2.7; 95% CI: 2.4–3.1), and total CV hospitalizations (OR = 2.3; 95% CI: 2.0–2.5) |
|
Valizadeh et al |
2015 |
110 women—abnormal glucose levels and metabolic syndrome; 1–6 y prior GD |
36 (32.7%)—type2 DM, 11 (10%)—impaired fasting glucose or impaired glucose tolerance, and 22 (20%)—metabolic syndrome |
|
Huang et al |
2016 |
Meta-analysis and prospective cohort |
Prediabetes—↑CVD/CHD and mortality: impairment—glucose tolerance, fasting glucose < 5.6 mmol/L/HbA1C (39 mmol/mol) and ↑HbA1C (39–7 mmol/mol) |
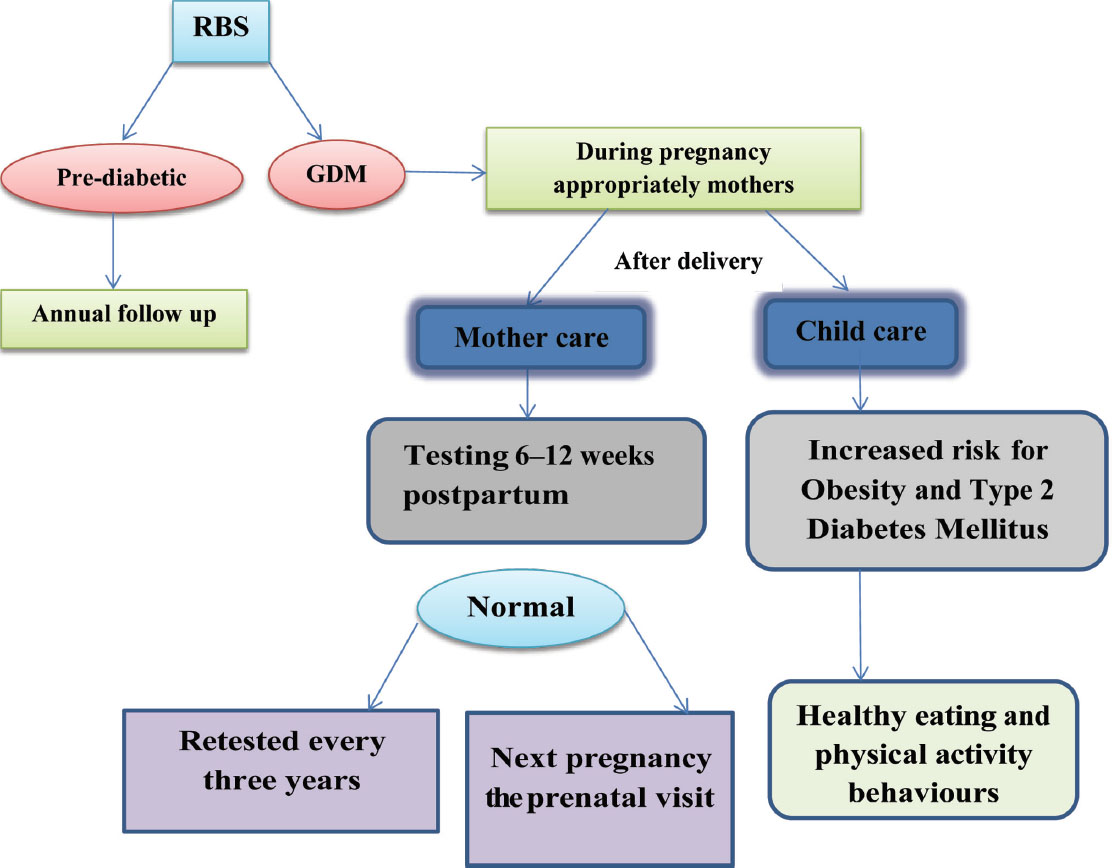
As prevention is better than cure, the American College of Obstetricians and Gynaecologists (ACOG) joined a program “Call for Action” that is an initiative of the National Diabetes Education to provide better health outcomes of women with prior GDM and their children12 (Fig. 3).
-
Fig. 3 Follow-up of GDM patient (ACOG and the American Diabetes Association recommend testing women recommendation).
Fig. 3 Follow-up of GDM patient (ACOG and the American Diabetes Association recommend testing women recommendation).
Preeclampsia
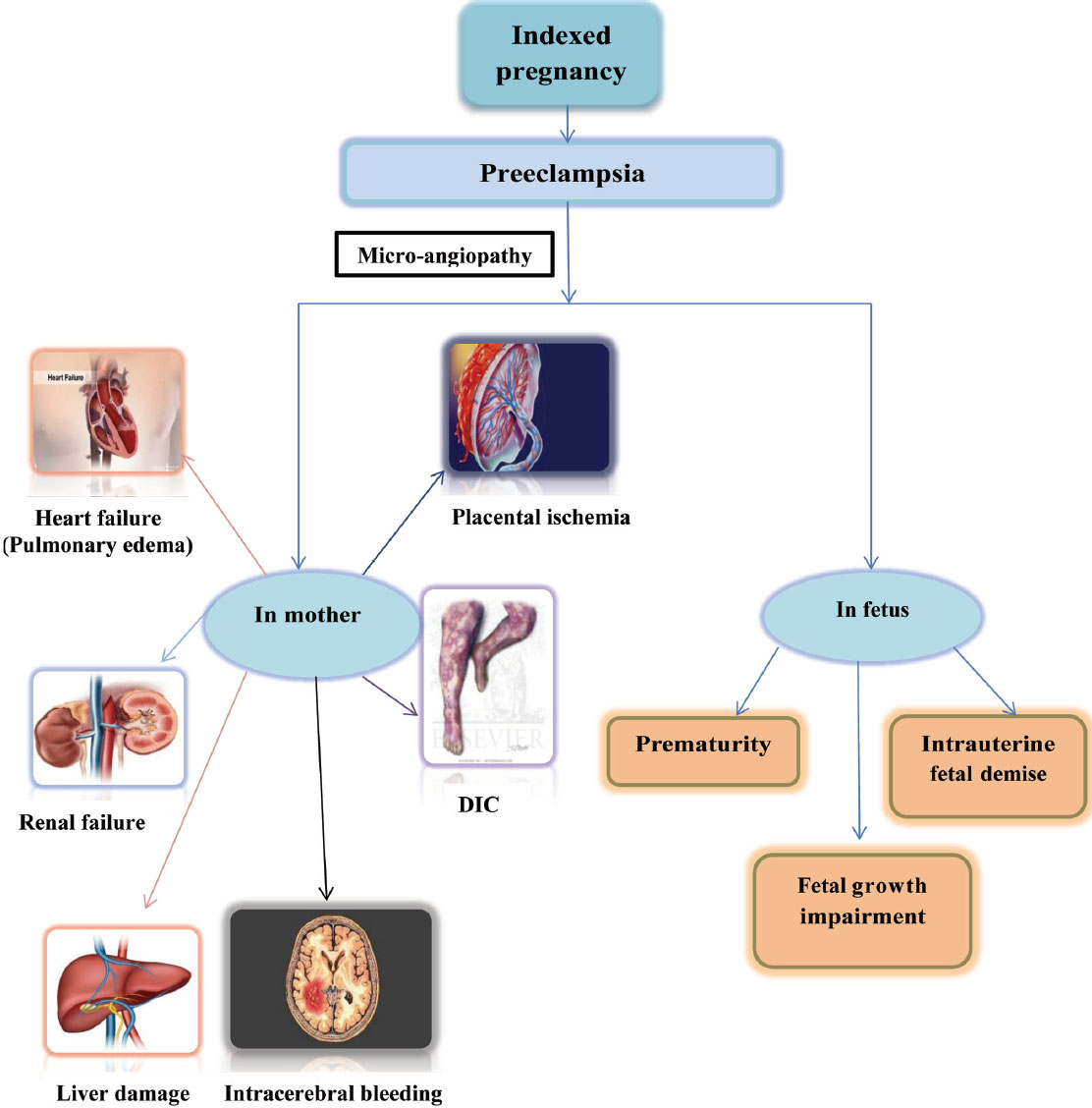
Preeclampsia induces micro-angiopathy that affects both the mother and fetus (Fig. 4). These changes of micro-angiopathy on the different organs do not dissolve after index pregnancy, which may be responsible for future maternal cardiovascular events (Fig. 5). The severity of development of long-term cardiovascular morbidity in the mother depends not only on having preeclampsia during pregnancy but also on how early it appears, birth weight of the baby, etc. (Fig. 6). The relative risk of future events in eclampsia and preeclamptic mothers is represented in Fig. 7. 13 14 In fact, gestational hypertension in the other futures of preeclampsia can also give rise in myocardial ischemia later in the life.
-
Fig. 4 Effects of preeclampsia during indexed pregnancy.
Fig. 4 Effects of preeclampsia during indexed pregnancy.
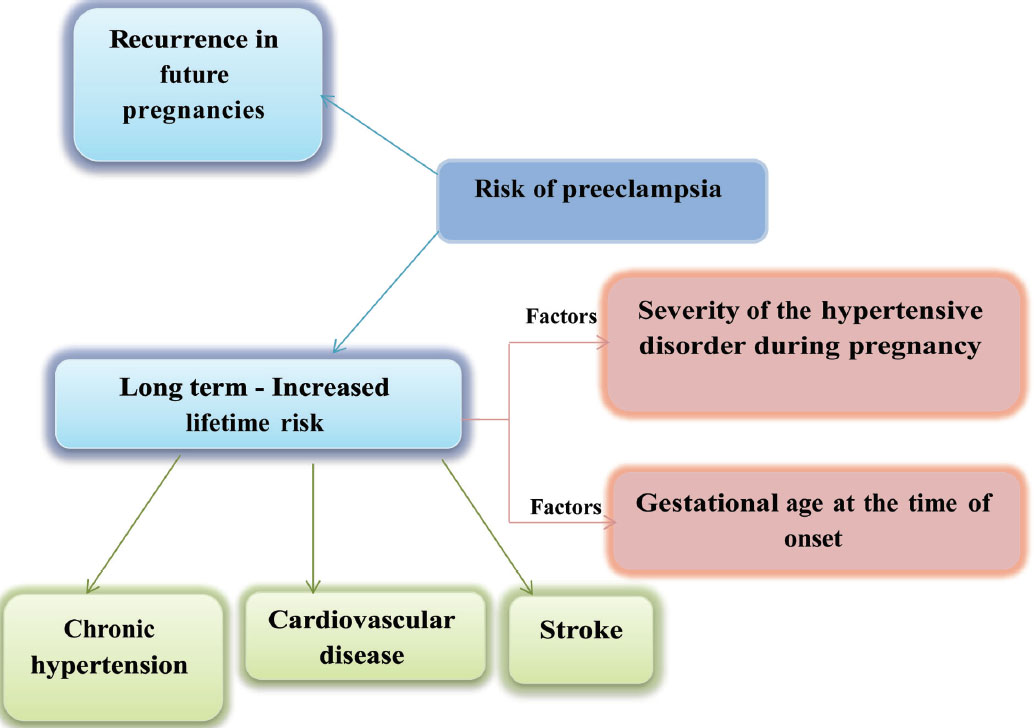
-
Fig. 5 Risk of preeclampsia.
Fig. 5 Risk of preeclampsia.
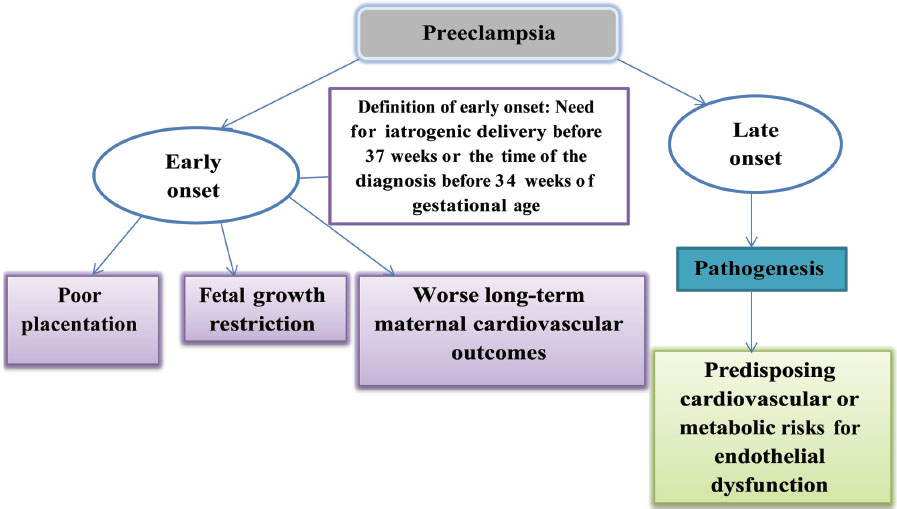
-
Fig. 6 Gestational age at the time of occurrence of preeclampsia.
Fig. 6 Gestational age at the time of occurrence of preeclampsia.
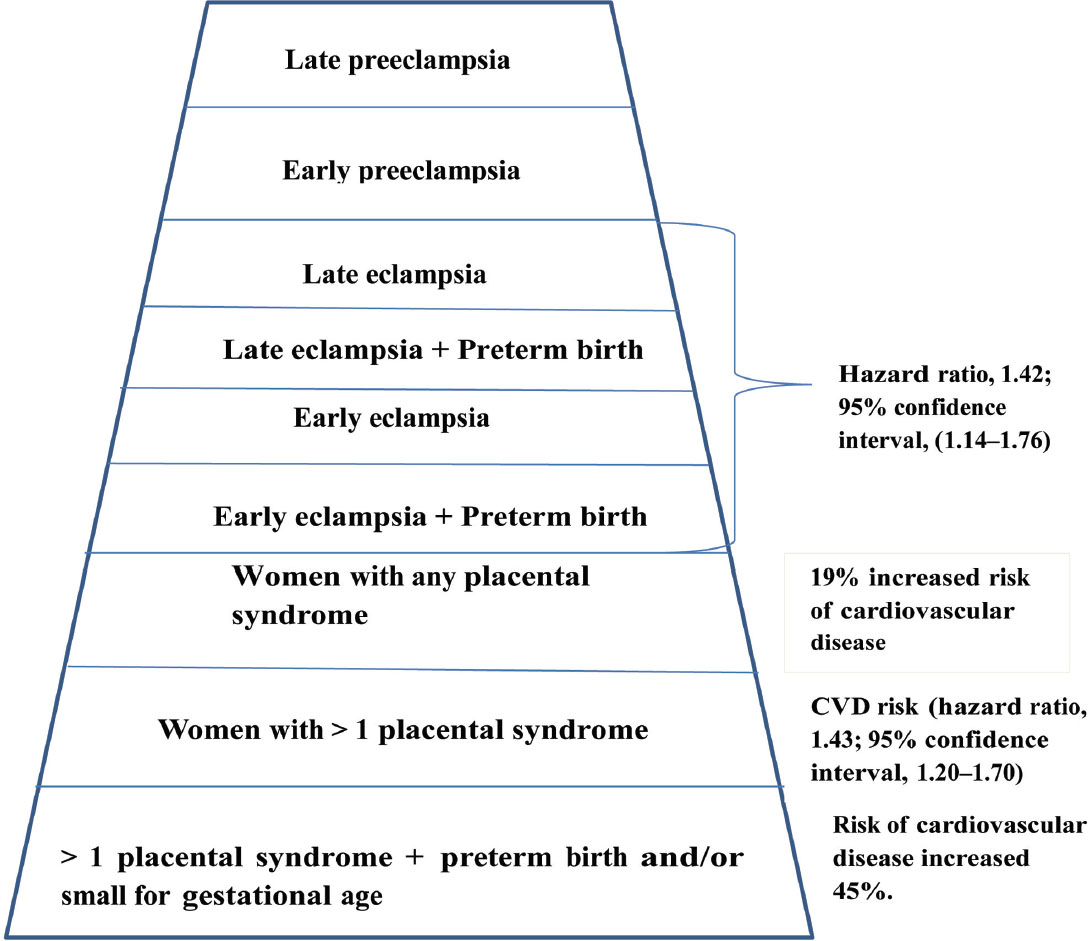
-
Fig. 7 Pyramid of relative risk of different stages of eclampsia for future cardiovascular disease.
Fig. 7 Pyramid of relative risk of different stages of eclampsia for future cardiovascular disease.
The long-term effects of preeclampsia in a mother are not only for the risk of hypertension but also for stroke, ischemic heart disease, venous thromboembolism, and overall mortality in the future (Fig. 8).
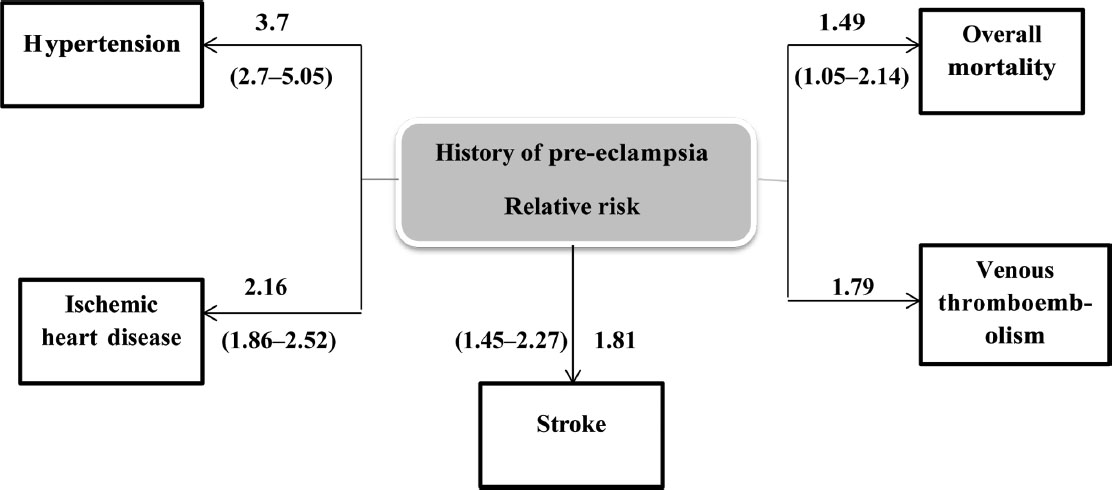
-
Fig. 8 Preeclampsia—subsequent cardiovascular disease.
Fig. 8 Preeclampsia—subsequent cardiovascular disease.
The fact that eclampsia can lead to late cardiovascular events are substantiated by multiple studies (Table 2).15 16 17 18 19 20 21 22 23
|
Study author |
Year |
Results |
|---|---|---|
|
Abbreviations: BMI, body mass index; CRP, C-reactive protein; CV, cardiovascular; CVD, cardiovascular disease; GENOA, Genetic Epidemiology Network of Arteriopathy; LV, left ventricular. |
||
|
McDonald et al |
2013 |
Women with a history of severe preeclampsia had higher rates of previous CVD than women with nonsevere preeclampsia or women without preeclampsia (87, 72, and 72%, p = 0.0019). Even after accounting for CV risk factors including albuminuria, a history of severe preeclampsia was independently associated with a 3-fold higher risk of CVD. |
|
Brown et al GENOA study |
2013 |
A history of hypertension in pregnancy is associated with elevated CRP levels later in life, independent of traditional CVD risk factors and BMI. |
|
Brown et al |
2013 |
Women diagnosed with history of preeclampsia were at increased risk of future CV or cerebrovascular events, with an estimated doubling of odds compared with unaffected women. |
|
Melchiorre et al |
2014 |
The relative risk of developing hypertension within 2 y of birth, even after adjusting for confounding risk factors, was increased 15-fold if LV abnormalities persisted. The higher prevalence of stage B heart failure in preterm than in term preeclampsia had a higher risk of subsequent congestive heart failure and ischemic cardiac diseases compared with women with term preeclampsia or normal pregnancy |
|
Veerbeek et al |
2015 |
Compared with women with late-onset preeclampsia and pregnancy-induced hypertension, women with previous early-onset preeclampsia had significantly higher fasting blood glucose (5.29 vs. 4.80 and 4.83 mmol/L), insulin (9.12 vs. 6.31 and 6.7 uIU/L), triglycerides (1.32 vs. 1.02 and 0.97 mmol/L), and total cholesterol (5.14 vs. 4.73 and 4.73 mmol/L). Almost one-half of women with early-onset preeclampsia developed hypertension, as opposed to 39% and 25% of women in the pregnancy-induced hypertension and late-onset preeclampsia groups, respectively. |
|
Weissgerber et al |
2016 |
A sibling history of hypertension in pregnancy was also associated with an increased risk of hypertension in brothers and unaffected sisters, whereas an increased risk of CV events was observed in brothers only. These results suggested that familial factors contribute to the increased risk of future hypertension in women who had hypertension in pregnancy. |
Few authors suggested that as early preeclampsia is associated with subsequent development of significant events, it is worth starting the preventive measures immediately after delivery. Few studies have concentrated on another cardiovascular risk (CVR) in preeclamptic women, which are mentioned in Table 3.24 25 26 27
|
Study |
Year |
Results |
|---|---|---|
|
Stekkinger et al |
2009 |
The metabolic syndrome was present in 15–25% of women after early-onset vascular-complicated pregnancy and in 10–14% of women after late–onset disease. |
|
Kessous et al |
2015 |
Patients with preeclampsia had significantly higher cumulative incidence of atherosclerotic–related hospitalizations and had an increased risk of cardiomyopathy during the peripartum period |
|
Behrens et al |
2016 |
Women with a history of hypertensive disorders of pregnancy had significantly increased rates of cardiomyopathy. These increases persisted > 5 y after the latest pregnancy. |
|
Black et al |
2016 |
2.36 and 2.48 times as likely, respectively, to develop pre-HTN/HTN (hypertension) in the year after delivery as those without pregnancy-related HTN. History of preeclampsia is also associated with an increased risk of future metabolic syndrome. |
Studies mentioned in Table 4 proved that preeclampsia increases not only CVR but other organ system disorders also.28 29 30 31
|
Study |
Year |
Results |
|---|---|---|
|
Aukes et al |
2012 |
History of preeclampsia was a risk marker for early cerebrovascular damage. They noted that formerly eclamptic women demonstrate cerebral white matter lesions (WMLs) several years following the index pregnancy. |
|
Weigman et al |
2012 |
Vision-related quality of life (QOL): Composite scores were significantly lower in formerly eclamptic women than in control participants (p < 0.01 for composite scores). |
|
Beharier et al |
2016 |
Women who had preeclampsia had a significantly higher incidence of long-term ophthalmic morbidity such as diabetic retinopathy and retinal detachment. In addition, a positive linear correlation was found between the severity of preeclampsia and the prevalence of future ophthalmic morbidities (0.3 vs. 0.5 vs. 2.2%, respectively). |
|
Postma et al |
2016 |
Formerly preeclamptic women reported cognitive dysfunction but did not exhibit overt cognitive impairment when objectively tested on average 6 y following their pregnancy. The presence of WML was not related to objective or to subjective cognitive impairment, anxiety, and depressive symptoms. |
Pathophysiology of Preeclampsia Leading to Cardiovascular Risk
There are a few common factors for both preeclampsia and CVD. Because of this common association, these preeclamptic patients develop CVD subsequently (Fig. 9).32
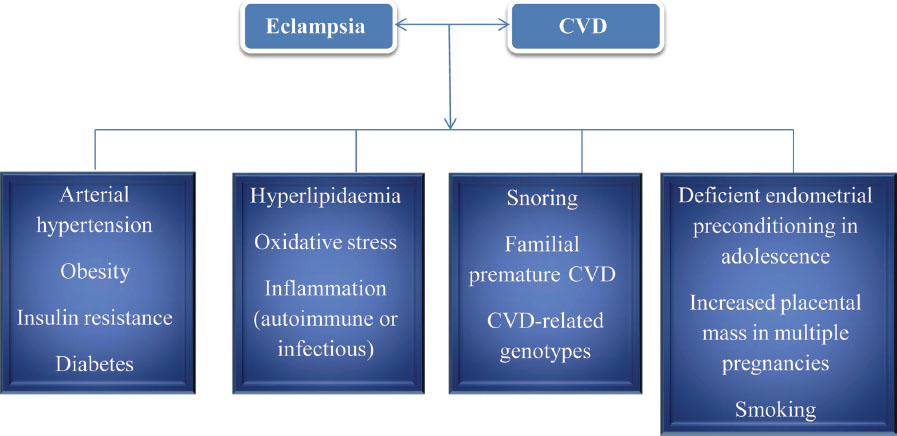
-
Fig. 9 Common factors for eclampsia and CVD.
Fig. 9 Common factors for eclampsia and CVD.
The main mechanism of preeclampsia leading to CVR later in the life is due to the endothelial dysfunction induced during pregnancy (Fig. 10).23

-
Fig. 10 Pathophysiology of complicated pregnancy leading to long-term maternal effects.
Fig. 10 Pathophysiology of complicated pregnancy leading to long-term maternal effects.
Preterm Deliveries
Preterm births were categorized as late (35–36 weeks), moderate (33–34 weeks), or extreme (≤32 weeks), and as spontaneous or indicated. In the previous section of preeclampsia, the effect of preterm delivery and of low-birth-weight baby was discussed. Preterm delivery with preeclampsia also has the risk of having a cardiac event later in the life. Studies supporting this are mentioned in Table 5.2 33 34 35 36
|
Study author |
Year |
Results |
|---|---|---|
|
Kessous et al |
2013 |
A linear association was found between the number of previous preterm delivery (PTD) and future risk of cardiovascular hospitalizations (5.5% for ≥ 2 PTDs; 5% for 1 PTD vs. 3.5% in the control group; p < 0.001). The association remained significant for spontaneous vs. induced PTD and for early (< 34 wk) and late (34 wk to 36 wk 6 days’ gestation) PTD. |
|
Robbins et al |
2014 |
Compared with women who had term deliveries, women with any history of PTB had increased risk of cardiovascular disease (CVD) morbidity (variously defined; adjusted hazard ratio [aHR] ranged from 1.2–2.9), ischemic heart disease (aHR, 1.3–2.1), stroke (aHR, 1.7), and atherosclerosis (aHR, 4.1). |
|
Ngo et al |
2015 |
aHR of CVD among women who ever had a preterm birth was 1.78 (1.61–1.96). Associations were greater for extreme (aHR = 1.98 [1.63–2.42]) and moderate (aHR = 2.06 [1.69–2.51]) than late preterm birth (aHR = 1.63 [1.44–1.85]), for indicated (aHR = 2.04 [1.75–2.38]) than spontaneous preterm birth (aHR = 1.65 [1.47–1.86]), and for having ≥ 2 (aHR = 2.29 [1.75–2.99]) than having 1 preterm birth (aHR = 1.73 [1.57–1.92]). |
|
Catov et al |
2016 |
The relative hazard (95% confidence interval [CI]) for metabolic syndrome was 1.52 (1.22–1.88) for women with preterm compared with term births. |
|
Pariente et al |
2016 |
Women with either spontaneous or indicated PTD had higher rates of renal-related hospitalizations (0.2% vs. 0.1%; odds ratio [OR] = 2.6; 95% CI: 1.7–3.9; p < 0.001 and 0.5% vs. 0.2%; OR = 3.41; 95% CI: 1.7–6.5; p < 0.001, respectively). |
Placental Abruption
Placental abruption is a condition with microvascular disturbance and leads to long-term effects on the mother’s health. The type of future cardiac diseases predisposed by placental abruption is mentioned in Fig. 11. In Table 6,37 38 39 40 studies on placental abruption are mentioned.
|
Study author |
Year |
Results |
|---|---|---|
|
Pariente et al |
2014 |
Compared with 46,932 women who delivered during the same period, the cardiovascular case fatality rate for the placental abruption group was 13.0% vs. 2.5% (p < 0.001). Placental abruption remained an independent risk factor for long-term maternal cardiovascular mortality (adjusted hazard ratio (HR) = 4.3; 95% confidence interval [CI]: 1.1, 18.6). |
|
Arazi et al |
2015 |
Placental abruption, even though considered a part of the “placental syndrome” with possible vascular etiology, was not found to be a risk factor for long–term maternal renal complications. |
|
DeRoo et al |
2016 |
Women with placental abruption in first pregnancy had an increased risk of cardiovascular death CVD death (HR ratio 1.8; 95% CI: 1.3, 2.4). Results were essentially unchanged by excluding women with pregestational hypertension, preeclampsia, or diabetes. Women with placental abruption in any pregnancy also had a 1.8–fold increased risk of CVD mortality (95% CI: 1.5, 2.2) compared with women who never experienced the condition. |
|
Ananth et al |
2017 |
CVD mortality rates in women with and without abruption were 0.9 and 0.3 per 10,000 person-years, respectively (adjusted HR 2.7, 95% CI: 1.5, 5.0). The corresponding CVD morbidity complication rates were 16.7 and 10.0 per 10,000 person-years, respectively (HR 1.5, 95% CI: 1.4, 1.8). |
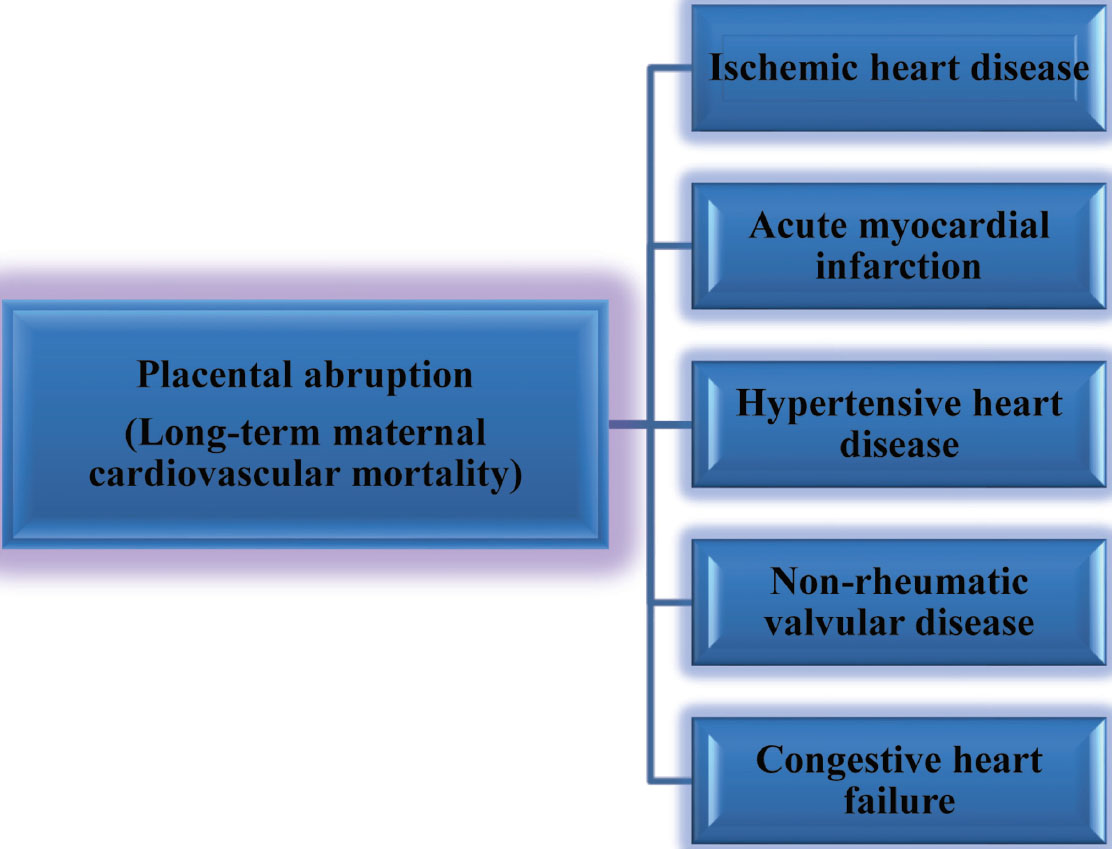
-
Fig. 11 Placental abruption—subsequent cardiovascular disease.
Fig. 11 Placental abruption—subsequent cardiovascular disease.
Stillbirth and Recurrent Miscarriages
A recurrent miscarriage in the mother is an indication to check for collagen vascular disorders. However, Pariente et al and Kessous et al have shown that recurrent miscarriages and stillbirths in women will predict the future cardiovascular events41 42 (Table 7).
|
Study author |
Year |
Results |
|---|---|---|
|
Pariente et al |
2014 |
After stillbirth, women had a significantly higher cumulative incidence of cardiovascular and renal morbidity and cardiovascular and renal hospitalizations, and had higher rates of simple and complex cardiovascular events. A significant stepwise increase was found between the number of stillbirths and future risk for cardiovascular morbidity. |
|
Kessous et al |
2014 |
Women with a history of recurrent pregnancy loss (RPL) had higher rates of renal and cardiovascular morbidity. Using a Cox proportional hazards model, adjusted for confounders such as preeclampsia, diabetes mellitus, obesity, and smoking, a history of RPL remained independently associated with cardiovascular hospitalizations. |
Maternal Obesity during Pregnancy
Maternal obesity during pregnancy will lead to obesity and related complications later in life. Reduction of even a few kilograms in weight, even for a shorter duration, has good long-term effects. Sasson et al concluded that obesity during pregnancy is an independent risk factor for long-term ophthalmic complications such as diabetic retinopathy.43 According to the 2016 Action for Health in Diabetes Study Group reports, weight loss in the overweight/obese individuals with type 2 diabetes had significant improvements in hemoglobin A1C (HbA1C), systolic blood pressure, high-density lipoprotein (HDL) cholesterol, and triglycerides (p ≤ 0.02).44
Women with Small for Gestational Age Neonate
Delivery of a small for gestational age (SGA) infant was one of the risk factors for the future maternal health. Parient et al45 and Almasi et al3 state that the delivery of an SGA neonate may lead to long-term complex cardiovascular events, including congestive heart failure, hypertensive heart and kidney disease, and acute cor pulmonale (odds ratio [OR] = 2.3; 95% confidence interval [CI]: 1.3–4.4; p = 0.006), and also long-term cardiovascular mortality (OR = 3.4; 95% CI: 1.5–7.6; p = 0.006).
Miscellaneous
-
Transient hypothyroidism in pregnancy can antedate CVD in later age.
-
Peripartum cardiomyopathy—the detailed discussion was given in a separate review (See Heart Failure in Pregnancy, p. 161 this issue).
Table 8 shows the diseases during pregnancy and their later development of cardiovascular and other organ involvements.46 47
|
Type of abnormality |
Cardiac (HF + CAD – microvascular dysfunction, obstructive lesions, AMI, coronary calcification) |
Thromboembolic |
Ophthalmology |
Renal |
Stroke |
Cor pulmonale |
Others (cancer) |
|---|---|---|---|---|---|---|---|
|
Abbreviations: AMI, acute myocardial infarction; CAD, coronary artery disease; GDM, gestational diabetes mellitus; HF, heart failure; SGA, small for gestational age. |
|||||||
|
GDM |
Yes |
No |
No |
No |
Yes |
No |
No |
|
Preeclampsia |
Yes |
Yes |
No |
No |
Yes |
No |
No |
|
Preterm deliveries |
Yes |
No |
No |
Yes |
Yes |
No |
No |
|
Abruption placenta |
Yes |
No |
No |
No |
No |
No |
No |
|
Maternal obesity |
No |
No |
Yes |
No |
No |
No |
No |
|
Women with SGA neonates |
Yes |
No |
No |
Yes |
No |
Yes |
No |
|
Preeclampsia and eclampsia |
Yes |
Yes |
No |
Yes |
Yes |
No |
No |
Preventive Measures
As many women come to regular checkup during pregnancy, it is an ideal time to detect the women who are at risk for the future CVD and implement at the same time the primary preventive strategies early such as health monitoring, lifestyle modifications, and other interventions, that will help reduce the burden of CVD (Fig. 12).48
Even though many obstetricians and gynecologists are aware of the fact that the risk of CVDs after preeclampsia is high, during the follow-up these women are not counseled and informed for preventive measures. By the implementation of the current guidelines both by obstetrician and cardiologists, these deficiencies can be overcome.49
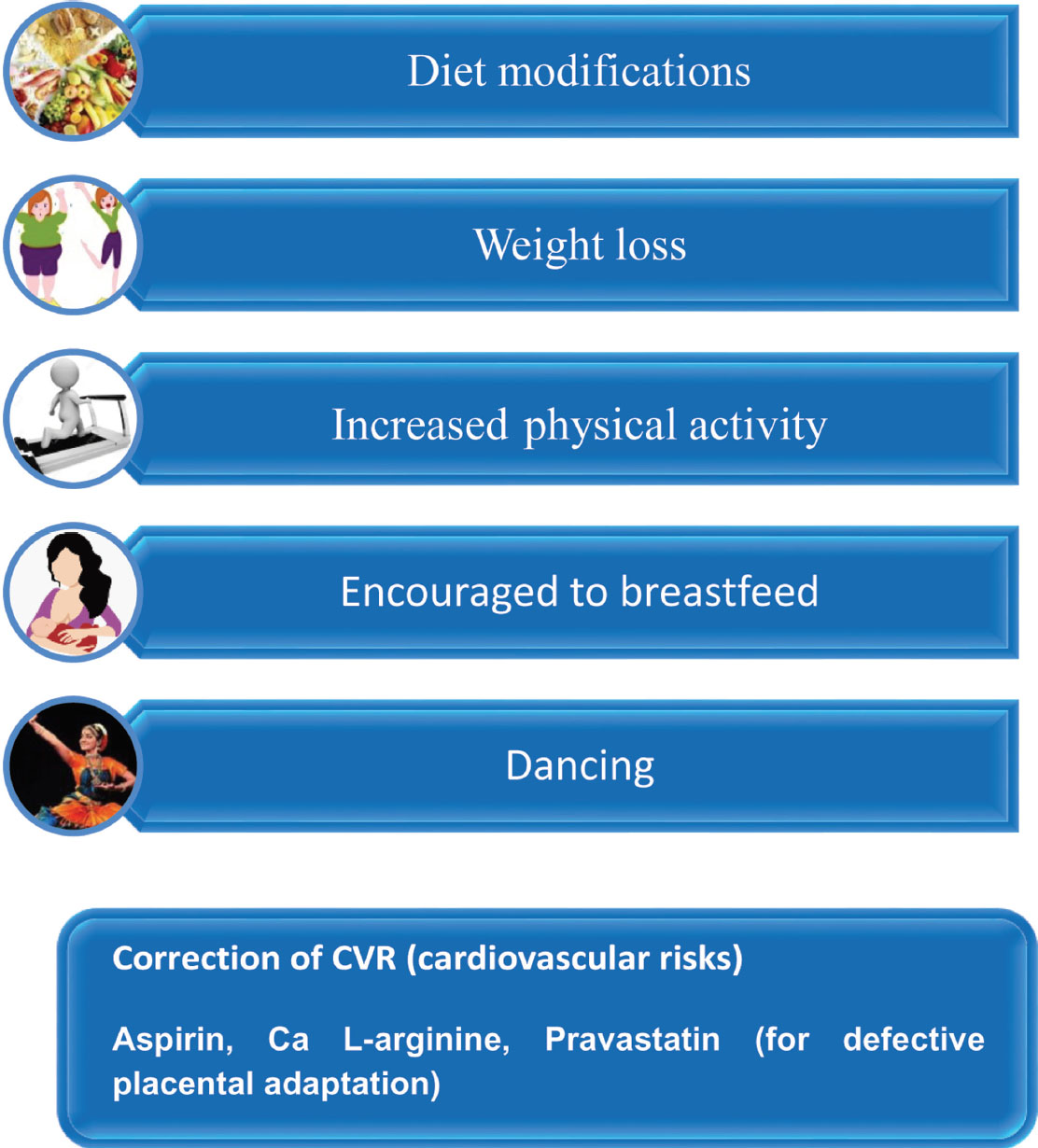
-
Fig. 12 Recommendations for prevention of long-term risk for mother.
Fig. 12 Recommendations for prevention of long-term risk for mother.
Conclusion
In women with a moderate or high risk of complications during pregnancy (modified World Health Organization [mWHO] II–III, III, and IV), prepregnancy counseling and management during and around delivery should be performed in an expert center by a multidisciplinary tea the pregnancy heart team.50 Follow them subsequently annually along with modification of CVR.
References
- Long-term effects of pregnancy complications on maternal health: a review. J Clin Med. 2017;6(08):76.
- [Google Scholar]
- Is preterm delivery an independent risk factor for long-term maternal kidney disease? J Matern Fetal Neonatal Med. 2017;30:1102-1107.
- [Google Scholar]
- Association between delivery of small-for-gestational-age neonate and long-term maternal chronic kidney disease. J Matern Fetal Neonatal Med. 2016;29:2861-2864.
- [Google Scholar]
- Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157-160.
- [Google Scholar]
- Effect of pre-diabetes on future risk of stroke: meta analysis. BMJ. 2012;344(03):564.
- [Google Scholar]
- Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355(05):953.
- [Google Scholar]
- Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773-1779.
- [Google Scholar]
- Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol. 2011;118:71-78.
- [Google Scholar]
- The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004-4010.
- [Google Scholar]
- The risk factors and incidence of type 2 diabetes mellitus and metabolic syndrome in women with previous gestational diabetes. Int J Endocrinol Metab. 2015;13(02):1696.
- [Google Scholar]
- An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart. 2013;99:1118-1121.
- [Google Scholar]
- Promoting health after gestational diabetes: a National Diabetes Education Program call to action. Obstet Gynecol. 2012;119:171-176.
- [Google Scholar]
- Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Obstet Gynecol. 2017;216(05):523.e1-523.e7.
- [Google Scholar]
- Long term mortality of mothers and fathers after preeclampsia: population based cohort study. BMJ. 2001;323:1213-1217.
- [Google Scholar]
- Increased cardiovascular risk after preeclampsia in women with dysglycaemia. Diabet Med. 2013;30:e1-e7.
- [Google Scholar]
- Hypertension in pregnancy is associated with elevated C-reactive protein levels later in life. J Hypertens. 2013;31:2213-2219.
- [Google Scholar]
- Hypertension in pregnancy and future cardiovascular event risk in siblings. J Am Soc Nephrol. 2016;27:894-902.
- [Google Scholar]
- Cardiovascular disease risk in women with preeclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19.
- [Google Scholar]
- Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703-714.
- [Google Scholar]
- Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65:600-606.
- [Google Scholar]
- A history of preeclampsia is associated with a risk for coronary calcification 3 years later. Am J Obstet Gynecol. 2016;214(04):519.e1-519.e8.
- [Google Scholar]
- Association of remote hypertension in pregnancy with coronary artery disease: a case-control study. Hypertension. 2009;53(04):733-738.
- [Google Scholar]
- Endothelial dysfunction after pregnancy-induced hypertension. Int J Gynaecol Obstet. 2014;124(03):230-234.
- [Google Scholar]
- Long-term maternal atherosclerotic morbidity in women with preeclampsia. Heart. 2015;101:442-446.
- [Google Scholar]
- Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. JAMA. 2016;315:1026-1033.
- [Google Scholar]
- Hypertensive disorders first identified in pregnancy increase risk for incident prehypertension and hypertension in the year after delivery. J Hypertens. 2016;34:728-735.
- [Google Scholar]
- Early-onset preeclampsia and the prevalence of postpartum metabolic syndrome. Obstet Gynecol. 2009;114:1076-1084.
- [Google Scholar]
- Preeclampsia and future risk for maternal ophthalmic complications. Am J Perinatol. 2016;33:703-707.
- [Google Scholar]
- Cerebral white matter lesions, subjective cognitive failures, and objective neurocognitive functioning: a follow-up study in women after hypertensive disorders of pregnancy. J Clin Exp Neuropsychol. 2016;38:585-598.
- [Google Scholar]
- Preeclampsia and cardiovascular disease: interconnected paths that enable detection of the subclinical stages of obstetric and cardiovascular diseases [review] Integr Blood Press Control. 2017;10:17-23.
- [Google Scholar]
- An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209:368.e1-368.e8.
- [Google Scholar]
- History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol. 2014;210:285-297.
- [Google Scholar]
- Preterm birth and future risk of maternal cardiovascular disease—is the association independent of smoking during pregnancy? BMC Pregnancy Childbirth. 2015;15:144.
- [Google Scholar]
- Preterm delivery and metabolic syndrome in women followed from prepregnancy through 25 years later. Obstet Gynecol. 2016;127:1127-1134.
- [Google Scholar]
- Placental abruption as a significant risk factor for long-term cardiovascular mortality in a follow-up period of more than a decade. Paediatr Perinat Epidemiol. 2014;28:32-38.
- [Google Scholar]
- Placental abruption and long-term maternal cardiovascular disease mortality: a population-based registry study in Norway and Sweden. Eur J Epidemiol. 2016;31:501-511.
- [Google Scholar]
- Cardiovascular disease in relation to placental abruption: a population-based cohort study from Denmark. Paediatr Perinat Epidemiol. 2017;31:209-218.
- [Google Scholar]
- Is there an association between a history of placental abruption and long-term maternal renal complications? J Matern Fetal Neonatal Med. 2015;28:1641-1646.
- [Google Scholar]
- Is stillbirth associated with long-term atherosclerotic morbidity? Am J Obstet Gynecol. 2014;211:416.e1-416.e12.
- [Google Scholar]
- Recurrent pregnancy loss: a risk factor for long-term maternal atherosclerotic morbidity? Am J Obstet Gynecol. 2014;211:414.e1-414.e14.
- [Google Scholar]
- Obesity during pregnancy and long-term risk for ophthalmic morbidity—a population-based study with a follow-up of more than a decade. J Matern Fetal Neonatal Med. 2016;29:2924-2928.
- [Google Scholar]
- Wing RR, Espeland MA, Hazuda HP, et al; Action for Health in Diabetes (Look AHEAD) Study Group. Association of Weight Loss Maintenance and Weight Regain on 4-Year Changes in CVD Risk Factors: the Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care. 2016;39:1345-1355.
- [Google Scholar]
- Association between delivery of a small-for-gestational-age neonate and long-term maternal cardiovascular morbidity. Int J Gynaecol Obstet. 2013;123:68-71.
- [Google Scholar]
- Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-978.
- [Google Scholar]
- Incident coronary heart disease after preeclampsia: role of reduced fetal growth, preterm delivery, and parity. J Am Heart Assoc. 2017;6(03):4158.
- [Google Scholar]
- Preeclampsia and long-term risk of cardiovascular disease: what do obstetrician-gynecologists know? BMC Pregnancy Childbirth. 2013;13:61.
- [Google Scholar]
- 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165-3241.
- [Google Scholar]







