Translate this page into:
Coronary Artery Perforation
Jyotsna Maddury, MD, DM, FACC Department of Cardiology, Nizam’s Institute of Medical Sciences (NIMS) Punjagutta, Hyderabad, Telangana 500082 India janaswamyjyotsna@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Percutaneous coronary intervention (PCI) is considered as the standard treatment of obstructive coronary artery disease in indicated patients. Even though PCI gives symptomatic angina improvement, but associated with serious complications like coronary artery perforation (CAP), the incidence is quite low. With the more complex lesions for successful angioplasty, different devices are required, which in turn increase the incidence of CAP in these patients. Here we review the classification, incidence, pathogenesis, clinical sequela, risk factors, predictors, and management of CAP in the current era due to PCI.
Keywords
coronary artery perforation
percutaneous coronary intervention
coronary artery perforation
management of coronary perfusion
Introduction
Percutaneous coronary intervention (PCI) for obstructive coronary artery diseases is accepted and has standardized procedure with minimal complication rates, including iatrogenic coronary artery perforation (CAP). Although angiographically significant coronary artery dissection is known to occur in up to 30% of all conventional balloon angioplasties,1 2 coronary perforation has been reported to occur in 0.3 to 0.6% of all patients undergoing PCI.3 4 5 6
Previous studies mentioned that predilatation before stenting predisposes for these complications, but subsequent studies disprove it. Increased incidence of CAP was reported with different coronary devices like atherectomy or rotablation, as more complex coronary lesions are being stented now. Here we review the incidence, causes, clinical sequela, and management of coronary perforation in the current era.7
Definition of Coronary Artery Perforation
CAP is defined as an anatomical breach in the wall of a coronary vessel due to the penetration of the three layers of the vessel wall, resulting in extravasation of blood or dye into the pericardium, myocardium, or adjacent cardiac chamber or vein.8
Consequences of Coronary Artery Perforation
Consequences of CAP depend on the location and severity. Location wise, if CAP occurs to the right or left ventricle, if not massive, then usually no immediate clinical consequences occur. If CAP occurs into the myocardium, myocardial hematoma occurs. If CAP occurs into the pericardium, then cardiac tamponade may occur. The severity of CAP was classified by Ellis et al (mentioned subsequently).
The mechanism of balloon angioplasty is by producing localized microdissections in the media and plaque fracture, not extending into the deeper layers of the arterial wall. CAP occurs when these dissections become extensive and penetrate through the vessel wall.
Incidence of Coronary Perforation
With standard simple PCI, the incidence of CAP is 0.1%.9 10 CAP incidence increase with the usage of GP IIb/IIIa inhibitors.11 12 13 14 Different incidences in different studies may be due to the difference in the definition of CAP, CTO intervention, and more aggressive debulking strategies for complex PCI success. Ajluni et al, in their large retrospective analysis, CAP reported in 0.4% of the PCIs.3
Predictors and Causes of CAP
Mainly the factors which lead to CAP are:
-
Patient-related factors
-
Procedure-related factors
-
Adjuvant therapy-related factors
-
Lesion-related factors
-
Stent-related factors
-
Balloon-related factors (Details included in the Procedure-related factors)
1. Patient-Related Factors
Risk factors for CAP include female gender, old age, non-ST (stent thrombosis) MI (myocardial infarction), lesion complexity, chronic total occlusion (CTO) intervention, no. of stents, and hypertension. CAP reported was 46% in female versus 26% in males, which was statistically significant (p = 0.001). Increased incidence in women may be due to the old age group and small size of the coronary arteries. Additional risk factors include the lower baseline creatinine clearance, previous coronary artery bypass grafting (CABG),15 history of congestive heart failure, and multivessel coronary artery disease.16 17 18 19
2. Procedure-Related Factors
CAP can occur at different stages of the procedure, along with the gadgetries used.
-
Type of guidewire and guidewire advancement.
-
A. Balloon/stent advancement.
-
B. Balloon/stent inflation.
-
C. Oversizing of the stent or ruptured balloon.
-
D. Improper position of the stent or balloon.
-
E. Type of balloon or stent.
A. Type of Guidewire and Guidewire Advancement
Heavy-weight and hydrophilic guidewires usage increases CAP incidence.20 Pressure wires used for fractional flow reserve (FFR) estimation are stiffer and less flexible wires than routine coronary wires, which require careful manipulation in complex or tortuous vessels. In Fig. 1, CAP occurred in proximal LCX when pressure wire was kept in LCX for equalization, before testing the FFR of left anterior descending artery (LAD) (Fig. 1).

-
Fig. 1 Pressure wire produced CAP. (a) Basal left angiogram, (b) Small extravasation of contrast from prox LCX in LAO caudal view when pressure entered the LCX, (c) Proximal LCX perforation in RAO caudal view, (d) After Balloon dilatation—CAP healed. CAP, coronary artery perforation. LAO, left anterior oblique; LCX, left circumflex; RAO, right anterior oblique.
Fig. 1 Pressure wire produced CAP. (a) Basal left angiogram, (b) Small extravasation of contrast from prox LCX in LAO caudal view when pressure entered the LCX, (c) Proximal LCX perforation in RAO caudal view, (d) After Balloon dilatation—CAP healed. CAP, coronary artery perforation. LAO, left anterior oblique; LCX, left circumflex; RAO, right anterior oblique.
During PCI, we have to pay attention to the distal tip of the guidewire position. Inadvertent advancement of guidewire more distally can lead to CAP. This type of excess free movement of guidewires occurs more frequently with hydrophilic wires.
B. Balloon/Stent Advancement
In tortuous and calcific lesions forcible advancement of the stent or the balloon can lead to CAP.
C. Balloon/Stent Inflation
Usually, either with the balloon or stent, dilation up to 1:1 ratio of the balloon or stent to an artery is considered ideal. Increase in this ratio is an important risk factor for CAP. The same thing was demonstrated by Ajluni et al in their study. 3 According to this study, CAP occurred more frequently with the balloon to artery ratio of 1.3 ± 0.3 (p < 0.001).3 Similarly, in a registry by Ellis et al, the balloon to artery ratio of those patients undergoing percutaneous transluminal coronary angioplasty (PTCA) complicated by perforation was 1.19 ± 0.17 versus 0.92 ± 0.16 for those without perforation (p = 0.03).5 This observation has been confirmed in another large randomized study. There was a two to threefold increase in severe dissection leading to vessel occlusion when the ratio was more than 1.1.1 5 Also, balloon rupture, particularly those associated with pinhole leaks (as opposed to longitudinal tears), may create high-pressure jets that increase the risk of dissection or perforation.
Another situation where CAP can happen is during high-pressure inflation of the balloon in resistant coronary lesions. To prevent this complication, new noncompliant balloons, where we can dilate up to 35 atm (for example OPN NC balloons), are available.
D. Oversizing of the Stent or Ruptured Balloon
During oversized balloon inflation, if a rupture of the balloon occurs, then chances of CAP are more. In Fig. 2, the oversized proximal implanted stent balloon was used to dilate the distal lesion, which produced CAP.
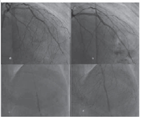
-
Fig. 2 Prolonged balloon inflation for Type II CAP. (a) Distal LAD lesion after stenting the prox LAD lesion, (b) CAP of distal LAD after same stent balloon dilatation (oversized balloon), (c) prolonged appropriate size balloon dilatation at CAP site, (d) stoppage of contrast leak. CAP, coronary artery perforation.
Fig. 2 Prolonged balloon inflation for Type II CAP. (a) Distal LAD lesion after stenting the prox LAD lesion, (b) CAP of distal LAD after same stent balloon dilatation (oversized balloon), (c) prolonged appropriate size balloon dilatation at CAP site, (d) stoppage of contrast leak. CAP, coronary artery perforation.
E. Improper Position of the Balloon or Stent
If a stent or balloon is passed over the unrecognized subintimal passage of the wire, which can happen especially in CTO lesions, this may cause severe dissection or even CAP. To prevent this, in a suspected subliminal location of the wire, it is better to perform intravascular ultrasound (IVUS) to confirm the wire position.
F. Type of Balloon or Stent
Cutting balloon usage and semicompliant balloon in resistant lesions may cause CAP. However, two studies differ saying cutting balloon does not predispose to CAP.21 22 Stiffer stents like covered stent may be difficult to negotiate in tortuous calcific lesions and may produce CAP during the forcible manipulations.
3. Adjuvant Therapy-Related Factors
The incidence of CAP is ~0.5 to 3% with the debulking devices like directional coronary atherectomy (DCA), excimer laser angioplasty, and rotational or extraction atherectomy.23 24 Even IVUS usage is also mentioned as one of the predictors of CAP, especially when intravascular ultrasound-guided PCI optimization was tried. In this case, Yukon stent of 3 × 18 mm is deployed in mid LAD lesion, and IVUS imaging was done to see the proper expansion of the stent. As there is underexpansion in the distal component of the stent on IVUS 3.25 NC balloon was used to dilate; immediate angiogram showed CAP which was controlled with prolonged balloon inflation and anticoagulation reversal (Fig. 3).
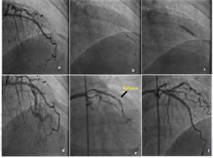
-
Fig. 3 IVUS-guided optimization of mid LAD stent produced-Type III CAP. (a) stent implantation, (b) IVUS passage, (c) re-balloon dilatation with bigger size balloon to optimize the stent expansion, (d) CAP in the stent, (e) healing of CAP after prolonged balloon dilatations, (f) sealing of the CAP. CAP, coronary artery perforation.
Fig. 3 IVUS-guided optimization of mid LAD stent produced-Type III CAP. (a) stent implantation, (b) IVUS passage, (c) re-balloon dilatation with bigger size balloon to optimize the stent expansion, (d) CAP in the stent, (e) healing of CAP after prolonged balloon dilatations, (f) sealing of the CAP. CAP, coronary artery perforation.
4. Lesion-Related Factors
A. Native lesion: Risk increases with complex lesion morphology such as chronic total occlusions (especially long-standing with bridging collaterals), angulated calcific lesions, tortuous vessels, bifurcation or ostial lesions, and eccentric or long lesions (>10 mm). The calcific lesion itself predisposes to CAP whether we use other adjuvant therapies or not..21 22 Small vessels, Type B2 or C lesions,25 the lesion in RCA or LCX, and eccentric lesions were the predictors for CAP in few studies.26
Saphenous vein graft (SVG) lesion: During CABG, usually pericardium is removed. Postsurgery, frequently an adhesion also develops. After surgery, adhesion of the remaining pericardium to the myocardium prevents the development of cardiac tamponade even when CAP occurs in a graft angioplasty. However, the CAP in the graft lesion is not always benign. Loculated effusions due to rapid extravasation of blood can occur, which are difficult to access for draining, but these collections may cause compression of cardiac chambers. Blood seepage from CAP may occur into the lung, causing hemoptysis or into the pleural cavity. On the contrary, not always pericardium is removed in all CABGs. Especially in young patients, many surgeons prefer to repair the pericardium, to facilitate second surgeries. Besides, some surgeons choose to repair the pericardium, as closed pericardium has been reported to paradoxically reduce postoperative tamponade after CABG surgery by protecting the heart from extrapericardial bleeding, in few studies. Where pericardium is closed or removed during CABG, CAP in graft lesions was associated with high mortality (22% at 30 days). The previous history of CVA, functional class of the patients, and the number of stents used were the predictors of CAP in graft angioplasty.
5. Stent-Related Factors
Stiff stents require high-pressure inflation for proper expansion, which can predispose for CAP.27 Another problem with stiff stents is these are less traceable, so if used in the tortuous vessel again perforation chances increase.
Sites of CAP
Coronary perforations can be made into:
-
Main vessel.
-
Distal vessel.
-
Branch vessel.
-
Collateral vessel.
Management of main and distal vessel perforations is discussed in the below section. Usually, septal collateral perforation produces myocardial hematoma. If recognized early and the procedure abandoned, then there may not be any consequences, but this requires observation like other site perforations to see for the increase in hematoma, then compression effects may occur. If epicardial collateral perforation occurs, then blood may seep into the pericardium.
Types of Perforation
Ellis et al evaluated a novel angiographic classification scheme for CAPs as a predictor of outcome.1 5 In a multicenter registry of 12,900 PCIs, 62 (0.5%) perforations were reported and categorized as:
Type I: Extraluminal crater without extravasation.
Type II: Epicardial fat or myocardial blush without contrast jet extravasation.
Type III: Extravasation through frank (>1 mm) perforation. or
Type III: “Cavity spilling” (CS) referring to Type III perforations with contrast spilling directly into either the left ventricle, coronary sinus, or another anatomic circulatory chamber.
The hypothesis that CAP may be the extension of the dissection is substantiated angiographically as the Ellis Type I perforation is identical to the previously described NHBLI (National Heart, Lung, and Blood Institute) Type C dissection.
Angiographically we can classify CAP as:
-
Free perforation—free contrast extravasation into the pericardium (Ellis Type III); or
-
Contained perforation—when contrast staining is seen around the vessel without free contrast leak.
Clinical Outcome after Perforation
Clinical outcome mainly depends on the severity of perforation. CAP with cardiac tamponade is closely associated with mortality.28 29 Even though aggressive management was done at the time of perforation, the complication rates are high. MI occurred in 16.7 to 50%, emergency surgery in 50%, and death in 9 to 19%.28 29 Late complications like pseudoaneurysm are more frequently associated with DCA or cutting balloon usage.28
According to Ajluni et al, contained perforation (tamponade 6%, CABG 24%, death 6%) had lesser event rate than free perforations (tamponade 20%, CABG 60%, death 20%). The incidence of MI or death, tamponade in Type I, II, III, III CS were 0, 8%; 14%, 13%; 19%, 63%; 0, 0, respectively. Type III CS was associated with no event rate. Twenty percent of the CAP was due to guidewire, and 80% was during or after stent implantation.30
Management of Coronary Perforation
1. Balloon Inflation
First step, immediately after CAP is, appropriate size balloon to be inflated, either proximal to or at the CAP site, to occlude the vessel and thus prevents the further leakage of blood into the pericardium.
2. Reversal of Anticoagulation
Second, the reversal of the anticoagulation (especially heparin) with protamine is done. Our aim is to achieve an activated clotting time of less than 150 seconds.
A concern previously arose for anticoagulation reversal due to artery or stent thrombosis, which was disproved subsequently.31 In diabetic patients who were on protamine insulin injection, protamine administration should be avoided. If the patient is on GPI with abciximab before CAP, then it is better to give platelet concentrate. As tirofiban and eptifibatide GPIs have shorter half-lives, stoppage of those drugs is sufficient. There was no increased incidence of cardiac tamponade in those who received GPI.31 32 After acute CAP management, it is advisable to continue antiplatelet therapy, as this has resulted in rebleeding.11 Even in other studies, also GPI and bivalirudin were not shown as significant risk factors.33
3. Pericardiocentesis
A. Early cardiac tamponade: If early cardiac tamponade is there, then pericardiocentesis is mandatory.
B. Delayed tamponade: Guidewire-associated CAPs and complex lesions are more likely present with delayed rather than early cardiac tamponade. Still, the need for surgical assistance is less (5%).34
4. Prolonged Balloon Inflation
A little bit longer than CAP neck and equal size of the perforated artery balloon should be inflated at the CAP site for 10 minutes. The ischemic tolerance of the patient decides the duration of inflation and repeated inflations also need to be done till the perforation seals off or the ischemic duration tolerated by the patient. Many times, patients adapt to the more extended time of artery occlusion in subsequent dilations than in the first time occlusion time due to postischemic adaption..
To decrease the myocardial ischemia during long balloon inflation time, autoperfusion balloons can be used. This type of balloon allows blood to flow from the proximal segment of the inflated balloon from the side holes and the blood travels through the balloon, then perfuses through the distal segment of the coronary bed. Another method is microcatheter distal perfusion technique, in which microcatheter is used to perfuse the distal bed on another coronary wire.35
Most of the time, Type I or II perforations can be managed with prolonged balloon inflation. However, we have to observe the patient even after initial stabilization for progression to tamponade or delayed tamponade, especially in guidewire-related perforations.12
Longer duration balloon inflations were required, if the severity of the perforation is the higher grade (Type I vs. Type II: 44 ± 37 minutes vs. 21 ± 13 minutes, respectively; p < 0.05 and Type II vs. Type III: 48 ± 37 minutes vs. 20 ± 13 minutes; p < 0.05).36 If the perforation is not sealed, then proceed to covered stent.37 38 39
5. Covered Stents for Proximal to Mid Coronary Perforations
Covered stents are preferred modality of treatment when CAP is in proximal or mid coronary arteries.37 38 39 Covered stents which were introduced initially for coronary aneurysms, are very useful to treat the CAP. Two requisites for the covered stent usage are perforation should be in proximal or mid of the vessel, and distal wire should be in the true lumen.16 40
The primary requisites for the usage of covered stents are the appropriate size of the perforated vessel, accessibility of the perforation site (in tortious and calcific vessels covered stent trackability becomes difficult), there should not be important big side branches, and the site of perforation should be very clear.41
Covered stents from different companies are available with different materials. Symbiot stent by Boston scientific is made of double-layered polytetrafluoroethylene (PTFE) on a modified self-expanding nitinol stent. Jostent by Abbott company (covered stent) is made up of single PTFE layer in-between two coaxial stainless steel stents. Nuvasc stent-graft from Cardiovasc is made up of a single layer of PTFE coated with synthetic material P-15 on a stainless steel stent. P-15 is a cell adhesion protein, promotes the endothelialization.
The major drawback of the above-covered stents is the trackability. To improve the traceability, newly pericardial covered stents are designed.42 Venous covered stents were reported to be used in SVG perforations.12 39 43 Even though autologous vein graft stents are available, but to prepare them in an emergency situation is not practical.39 Papyrus stent has easy trackability as electrospun polyurethane membrane is used. Thin layers of polyethylene terephthalate of mesh stent also is another trackable stent.44
Another problem with the covered stents is stent thrombosis in 5.7% and restenosis in 31.6% (angiographic) of the patients. So, it is advisable to give a longer duration of dual anti platelet therapy (DAPT).45
6. Alternatives to Covered Stents
A. In the absence of covered stents, bare-metal stents with narrow struts can be tried to seal the perforation.46
B. Steps to make a “covered stent” (sandwich stent) in cath laboratory “Sahoo’s method.”
(*Personal communication from Dr. Prasant Kr. Sahoo, Apollo Hospitals, Bhubaneswar, Odisha)
-
Choose your stent size as per your perforated artery size (say, e.g., perforated artery 3 mm).
-
Take two available stents on the shelf (Bare/DES):
-
Stent I: 3 × 28 mm (I being “inner stent”).
-
Stent O: 3 × 24 mm (O being “outer stent”).
-
-
Please note that “stent I” should be longer than “stent O” by at least 4 mm (Fig. 4a).
-
Preparation of stent O:
-
Cut both ends of stent O (3 × 24) with sharp scissors after inflating the stent O to 3 to 4 atm. While cutting both ends of stent O, load a stiff end of PTCA wire or the “stylet wire” (found inside every newly opened stent), from the proximal end of the stent O. One can also use sharp “surgical blade” to cut both ends of stent O (Fig. 4b).
-
Load this partially inflated cut stent O on a “wire” from the proximal end of the cut stent. (If you had loaded it on the stiff end of a PTCA wire, this small piece of cut wire would come out, and the newly prepared stent O will be loaded on the “stylet wire”).
-
So, now you have a “partially expanded” stent with a layer of “balloon” loaded on a wire. This newly prepared stent O has two layers: (1) metal outer layer and (2) balloon inner layer (Fig. 4c).
-
-
Now load stent I (3 × 28) on the newly prepared stent O with the help of the loading wire and position it so that partial part of stent I projects from the proximal and distal ends of the stent O (This is why stent L should be slightly larger than stent O) (Fig. 4d).
-
Crimp stent O on stent I, thereby preparing a “sandwich stent,” which is ready for use. This sandwich stent will have three layers ([1] Metal inner layer, [2] Balloon middle layer, [3] Outer metal layer—as illustrated below) (Fig. 4e).
-
Now load this newly prepared “covered stent” on the coronary guidewire and implant it with higher than nominal pressure across the perforated site. If there is still some leakage go to still higher pressures, so as to get proper apposition of the newly prepared “covered stent.” (The purpose of taking a longer inner stent is to prevent “seepage of blood” at the edges of the outer layer. Moreover, the edges of the outer stent can be “post dilated” with another new balloon with a slightly higher pressure than nominal, for better apposition in case there is any leakage.)
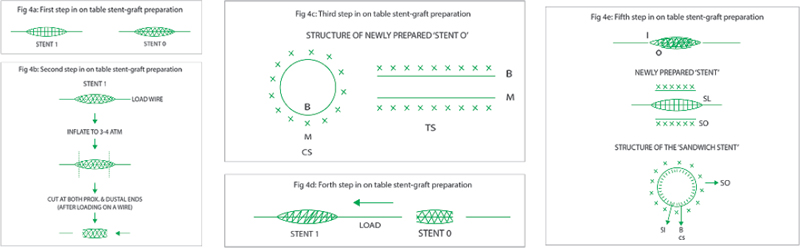
-
Fig. 4 (a) First step in on table stent-graft preparation. (b) Second step in on table stent-graft preparation. (c) Third step in on table stent-graft preparation. (d) Forth step in on table stent-graft preparation. (e) Fifth step in on table stent-graft preparation.
Fig. 4 (a) First step in on table stent-graft preparation. (b) Second step in on table stent-graft preparation. (c) Third step in on table stent-graft preparation. (d) Forth step in on table stent-graft preparation. (e) Fifth step in on table stent-graft preparation.
(Important corollary: A similar “peripheral covered stent” can be done using two renal stents for peripheral artery perforations, in case peripheral covered stents are not available on the shelf. This has been successfully done in a case by the author also.)
7. Methods to Treat Distal CAP
For distal perforations we can use gel foam or metal coils.47 48 Other embolization materials described in the literature are coagulated blood from the patient, thrombin, two-component fibrin-glue, collagen, transcatheter subcutaneous tissue delivery, cyanoacrylate liquid glue, denatured alcohol, tris-acryl gelatin microsphere, or polyvinyl alcohol particles and use of a local drug delivery catheter.47
-
Coils: Coils are a metallic wire with Dacron or wool as thrombogenic materials. We have to select the size of the coil, which should be bigger than the vessel perforated. The too big coil may dislodge in the proximal segment of the artery or too small one may embolize distally. These coils may be delivered through the guide catheter or more precisely exactly to the distal segment through microcatheter.48
Microcoils can be used for sealing of perforation without reversal of anticoagulation. If required, they can be coated with fiber or gel for thrombogenicity. The microcoils are made of platinum, so they are more radiopaque with less risk of thrombosis.49
-
Microspheres: These are hydrophilic nonabsorbable spherical particles. The size of these microspheres may range from 1 to 1,500μm and delivered to the site of perforation through microcatheter. These were tried mainly in collateral perforations. Safety of this method requires validation.50 51
-
Thrombin injection: Thrombin promotes fibrin formation due to its platelet activator property. Solutions or glue with thrombin or fibrinogen has to be delivered to the perforation site with microcatheter carefully or over the balloon, to prevent the spillage of the material proximally.52 53 54 55
-
Autologous blood clots: Major advantages of using autologous blood clots are easy availability, no cost, biocompatibility, and will be lysed automatically later. These blood clots are usually mixed with contrast media or saline, and then injected to the particular site.56
-
Fat embolization: Autologous subcutaneous fat advantage is the same as an autologous blood clot. The mechanism of thrombus generation by this fat is, it causes a physical barrier and prevents the blood leakage, in addition to its thrombogenic property. This fat is usually mixed with contrast for radiopacity.57 58
-
Miscellaneous: Other materials that have been used for embolization include synthetic glues, two-component adhesives made of fibrinogen and thrombin, collagen, polyvinyl alcohol particles, and protamine.54 55 59 Experience of the operator and availability of the embolic materials are the limitations in their usage for distal perforations.
8. Surgery
Surgical ligation of the vessel at perforation site and bypass graft to the distal vessel are required for severe CAP patients. When multiple stents are used along with subepicardial hematoma then it is better to seal the perforation site with additional Teflon or pericardial patch.60
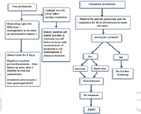
The algorithm to follow in CAP is mentioned in Figs. 5 and 6.
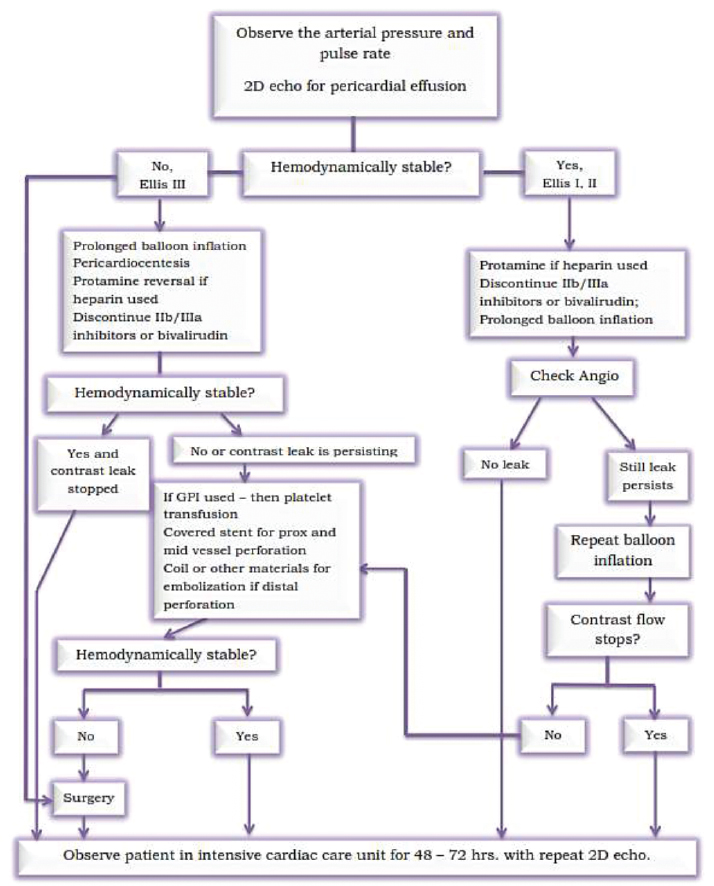
-
Fig. 5 First algorithm for the management of coronary perfusion.
Fig. 5 First algorithm for the management of coronary perfusion.
-
Fig. 6 Management of coronary perfusion—continuation.
Fig. 6 Management of coronary perfusion—continuation.
Diagnosis of Coronary Perforation
Coronary perforation can be easily diagnosed by coronary angiography and echocardiography, and it is usually accompanied by new episodes of chest pain, hemodynamic deterioration, and electrocardiographic changes.
Angiographic evidence of perforation is the presence of blush, jet, coronary sinus compression, and contrast in the pericardium.
When there is delayed post PCI hypotension then delayed tamponade should be suspected. This is more common in case of perforations induced by a guidewire or GP IIb/IIIa.37 61 Repeat echo after 24 hours is mandatory to detect the delayed pericardial collection, especially in the causes of distal perforation and covered stent usage.62
Contained Perforation and Pseudoaneurysm
Another important complication of CAP of contained variety is pseudoaneurysm (local vessel dilation >1.5 times when compared with the normal adjacent artery) formation. This can happen as early as 10 minutes to as late as 2 to 3 weeks. Even though there is no literature on the incidence of these pseudoaneurysms rupture, this needs careful follow-up, and may require surgery.63 This is the case of pseudoaneurysm after the CAP, subsequently treated with a covered stent (Fig. 7).
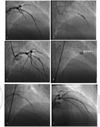
-
Fig. 7 Contained perforation—irregular bulge during balloon dilation gives a clue that some stent deformity occurs at that level. (a) Poststent deployment—LAO cranial; (b) Irregular balloon dilation at the overlap area of the stent; (c) Contained perforation; (d) Strut fracture; (e) Placement of a covered stent; (f) A good result after covered stent at the contained perforation site.
Fig. 7 Contained perforation—irregular bulge during balloon dilation gives a clue that some stent deformity occurs at that level. (a) Poststent deployment—LAO cranial; (b) Irregular balloon dilation at the overlap area of the stent; (c) Contained perforation; (d) Strut fracture; (e) Placement of a covered stent; (f) A good result after covered stent at the contained perforation site.
Prognosis after Treatment of CAP
Depending on the severity of vessel wall injury mortality also increases, it can be high (21.2%), and may result in periprocedural myocardial infarction in 34.0%. High all-cause mortality does not only occur in-hospital but also at 30 days (10.7%) and 1 year (17.8%).62 64
In a study, the in-hospital mortality was significantly high in tamponade group (7.7%) than without (4.3%). This tamponade group had a threefold increase in death on long-term follow-up.65 Mortality was found to be significantly higher in acute tamponade compared with delayed tamponade (59% vs. 21%, respectively; p = 0.04).66
This excess mortality may be related to underlying ischemia due to untreated coronary stenosis, side-branch loss with periprocedural MI, access site complications, major bleeding, and transfusion and risk of stent thrombosis, and restenosis with covered stent usage.15
Conclusion
CAP even though rare, is a dreaded complication of PCI. Best modality of treatment is prevention. This requires early detection and immediate attention on the cath laboratory table to treat CAP immediately. The preferred sequence of steps to be taken in the management of the CAP depend on the type of CAP.
Contained perforation may have a relatively benign course immediately in hospital than free perforations. Immediately after CAP, prolonged balloon inflation at CAP site, anticoagulation reversal, and pericardiocentesis should be done in case of proximal or mid coronary vessel perforation. If no improvement, then covered stent should be placed. In distal perforations, it is better to use coils or above said alternatives. Two-dimensional echocardiogram plays an important role not only in the early tamponade but also requires repetition after 24 hours. If all these measures fail, then plan for CABG. CAP patients require meticulous long-term follow-up as there are chances of pseudoaneurysm formation, covered stent thrombosis or stenosis, and more cardiovascular event rates.
Conflict of Interest
None declared.
References
- Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90(06):2725-2730.
- [Google Scholar]
- Coronary perforation during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade: an algorithm for percutaneous management. Catheter Cardiovasc Interv. 2001;52(03):279-286.
- [Google Scholar]
- Perforations after percutaneous coronary interventions: clinical, angiographic, and therapeutic observations. Cathet Cardiovasc Diagn. 1994;32(03):206-212.
- [Google Scholar]
- Aortocoronary dissection complicating a percutaneous coronary intervention. J Invasive Cardiol. 2003;15(02):89-92.
- [Google Scholar]
- Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Circulation. 1990;82(04):1193-1202.
- [Google Scholar]
- Transluminal coronary angioplasty complicated by coronary artery perforation. Cathet Cardiovasc Diagn. 1982;8(05):481-487.
- [Google Scholar]
- Coronary arterial rupture during coronary angioplasty. Am J Cardiol. 1983;51(05):902-904.
- [Google Scholar]
- Coronary artery perforation complicated by recurrent cardiac tamponade: a case illustration and review. Cardiovasc Revasc Med. 2017;18:S30-S34. 5S1
- [Google Scholar]
- Immediate and chronic results of cutting balloon angioplasty: a matched comparison with conventional angioplasty. Clin Cardiol. 1997;20(05):459-463.
- [Google Scholar]
- Initial experience with a hydrophilic-coated guidewire for recanalization of chronic coronary occlusions. Catheter Cardiovasc Interv. 2000;49(01):45-50.
- [Google Scholar]
- Coronary perforation during percutaneous coronary intervention. Int Heart J. 2007;48(01):1-9.
- [Google Scholar]
- Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J. 2002;66(04):349-356.
- [Google Scholar]
- Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2006;98(07):911-914.
- [Google Scholar]
- Coronary artery perforation in patients undergoing percutaneous coronary intervention: a single-centre report. Acute Card Care. 2009;11(04):216-221.
- [Google Scholar]
- Coronary perforation complicating percutaneous coronary intervention in patients with a history of coronary artery bypass surgery: an analysis of 309 perforation cases from the British Cardiovascular Intervention Society Database. Circ Cardiovasc Interv. 2017;10(09):e005581.
- [Google Scholar]
- Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart. 2002;88(05):495-498.
- [Google Scholar]
- Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2000;86(06):680-682.
- [Google Scholar]
- Coronary artery perforations in the contemporary interventional era. J Interv Cardiol. 2009;22(04):350-353.
- [Google Scholar]
- Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J. 2004;147(01):140-145.
- [Google Scholar]
- Primary coronary artery dissection observed at coronary angiography. Am J Cardiol. 1988;61(08):645-648.
- [Google Scholar]
- A word of caution on optimizing stent deployment in calcified lesions: acute coronary rupture with cardiac tamponade. Am Heart J. 1996;131(01):192-194.
- [Google Scholar]
- Benefit of intracoronary ultrasound in the deployment of Palmaz-Schatz stents. J Am Coll Cardiol. 1994;24(04):996-1003.
- [Google Scholar]
- Case report: a very large dissection in the left anterior descending coronary artery of a 56-year-old man. Cardiovasc Revasc Med. 2006;7(04):240-242.
- [Google Scholar]
- Review and hypothesis: the eosinophil and peripartum heart disease (myocarditis and coronary artery dissection)—coincidence or pathogenetic significance? Cardiovasc Res. 1997;33(03):527-532.
- [Google Scholar]
- Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations. Am J Cardiol. 2004;93(02):213-216.
- [Google Scholar]
- Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2009;104(12):1674-1677.
- [Google Scholar]
- Long-term follow-up of coronary artery dissection due to blunt chest trauma with spontaneous healing in a young woman. Intensive Care Med. 1996;22(05):450-452.
- [Google Scholar]
- Spontaneous coronary artery dissection associated with cocaine use: a case report and brief review. Cardiovasc Pathol. 2001;10(03):141-145.
- [Google Scholar]
- Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989;64(08):471-474. Jr
- [Google Scholar]
- The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol. 2004;16(06):257-301.
- [Google Scholar]
- Coronary artery perforation during percutaneous coronary intervention: incidence and outcomes in the new interventional era. J Invasive Cardiol. 2005;17(11):603-605.
- [Google Scholar]
- Outcomes of patients with coronary artery perforation complicating percutaneous coronary intervention and correlations with the type of adjunctive antithrombotic therapy: pooled analysis from REPLACE-2, ACUITY, and HORIZONS-AMI trials. J Interv Cardiol. 2009;22(05):453-459.
- [Google Scholar]
- Guidewire-induced coronary artery perforation and tamponade during PCI: in-hospital outcomes and impact on long-term survival. J Invasive Cardiol. 2014;26(08):371-376.
- [Google Scholar]
- Complications encountered in coronary chronic total occlusion intervention: prevention and bailout. Indian Heart J. 2016;68(05):737-746.
- [Google Scholar]
- Outcome of prolonged balloon inflation for the management of coronary perforation. J Cardiol. 2013;61(03):206-209.
- [Google Scholar]
- Di Mario C, Grube E, Colombo A. Emergency polytetrafluoroethylene-covered stent implantation to treat coronary ruptures. Circulation. 2000;102(25):3028-3031.
- [Google Scholar]
- Treatment of coronary artery perforations complicating percutaneous coronary intervention with a polytetrafluoroethylene-covered stent graft. Am J Cardiol. 2006;98(03):370-374.
- [Google Scholar]
- Coronary artery perforation following percutaneous coronary intervention. J Invasive Cardiol. 2016;28(03):122-131.
- [Google Scholar]
- Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv. 2010;75(05):708-712.
- [Google Scholar]
- Use of a novel pericardial covered stent to seal an iatrogenic coronary perforation. J Invasive Cardiol. 2009;21(10):E187-E190.
- [Google Scholar]
- Covered stent to treat saphenous venous graft perforation—a case report. Catheter Cardiovasc Interv. 2010;76(06):844-846.
- [Google Scholar]
- Successful sealing of a coronary artery perforation with a mesh-covered stent. J Invasive Cardiol. 2012;24(04):E80-E83.
- [Google Scholar]
- Results of the Jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc Interv. 2002;56(03):353-360.
- [Google Scholar]
- Coronary perforation due to sirolimus-eluting stent’s strut rupture with post-dilatation. Kardiol Pol. 2011;69(02):183-186, discussion 187.
- [Google Scholar]
- Use of stent grafts and coils in vessel rupture and perforation. J Interv Cardiol. 2008;21(01):86-99.
- [Google Scholar]
- Management of distal coronary perforations. J Invasive Cardiol. 2008;20(06):E187-E191.
- [Google Scholar]
- Is a metallic microcoil really a permanent embolic agent for the management of distal guidewire-induced coronary artery perforation? Korean Circ J. 2011;41(08):474-478.
- [Google Scholar]
- Cardiac tamponade due to coronary perforation during percutaneous interventions successfully treated with microspheres. Clin Res Cardiol. 2014;103(04):325-327.
- [Google Scholar]
- Huge coronary perforation during percutaneous intervention sealed by injection of polyvinyl alcohol microspheres. J Cardiovasc Med (Hagerstown). 2015;16:S130-S132. Suppl 2
- [Google Scholar]
- Successful treatment of distal coronary guidewire-induced perforation with balloon catheter delivery of intracoronary thrombin. Catheter Cardiovasc Interv. 2003;58(03):370-374.
- [Google Scholar]
- Intracoronary thrombin injection using a microcatheter to treat guidewire-induced coronary artery perforation. Cardiovasc Revasc Med. 2011;12(05):329-333.
- [Google Scholar]
- Delayed and repeated cardiac tamponade following microleak in RCA successfully treated with intra arterial sterile glue injection. Catheter Cardiovasc Interv. 2009;73(06):797-800.
- [Google Scholar]
- Closure of guide wire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv. 2007;70(02):237-240.
- [Google Scholar]
- Intracoronary autologous blood to seal a coronary perforation. Herz. 2001;26(02):157-160.
- [Google Scholar]
- Guidewire-induced coronary perforation successfully treated with subcutaneous fat embolisation: a simple technique available to all. Catheter Cardiovasc Interv. 2015;86(07):1186-1188.
- [Google Scholar]
- Management of guidewire-induced distal coronary perforation using autologous fat particles versus coil embolization. Catheter Cardiovasc Interv. 2017;89(02):253-258.
- [Google Scholar]
- Collagen embolization for the successful treatment of a distal coronary artery perforation. Catheter Cardiovasc Interv. 2009;73(03):332-335.
- [Google Scholar]
- Teflon felt wrapping repair for coronary perforation after failed angioplasty. Ann Thorac Surg. 2006;82(06):2312-2314.
- [Google Scholar]
- Successful treatment of a saphenous vein graft perforation with an autologous vein-covered stent. Catheter Cardiovasc Interv. 1999;48(04):382-386.
- [Google Scholar]
- Clinical characteristics and management of coronary artery perforations: a single.–.center 11.–.year experience and practical overview. J Am Heart Assoc. 2017;6(09):e007049.
- [Google Scholar]
- Occurrence of a saccular pseudoaneurysm formation two weeks after perforation of the left anterior descending coronary artery during balloon angioplasty in acute myocardial infarction. Catheter Cardiovasc Interv. 1999;47(03):341-346.
- [Google Scholar]
- Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol. 2011;27(06):843-850.
- [Google Scholar]
- Cardiac tamponade complicating coronary perforation during angioplasty: short-term outcomes and long-term survival. J Invasive Cardiol. 2013;25(10):486-491.
- [Google Scholar]
- Diagnosis, management, and clinical outcome of cardiac tamponade complicating percutaneous coronary intervention. Am J Cardiol. 2002;90(11):1183-1186.
- [Google Scholar]







